On the binding of auranofin to Prdx6 and its potential role in cancer cell sensitivity to treatment
IF 7.1
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
In this study, we demonstrate that ferroptosis is a component of the cell death mechanism induced by auranofin in HT-1080 cells, in contrast to the gold(III) compounds [Au(phen)Cl2]PF6 and [Au(bnpy)Cl2]. Additionally, we identify a potential role of Prdx6 in modulating the sensitivity of A-375 cells to auranofin treatment, whereas the gold(III) compounds evaluated here exhibit Prdx6-independent cytotoxicity. Finally, using mass spectrometry, we show that auranofin binds selectively to the catalytic Cys47 residue of Prdx6 in vitro under acidic conditions. No binding was observed with the C47S mutant or at neutral pH.
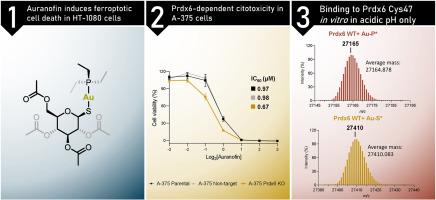
欧拉诺芬与 Prdx6 的结合及其在癌细胞对治疗敏感性中的潜在作用。
在这项研究中,我们证明了金(III)化合物[Au(phen)Cl2]PF6和[Au(bnpy)Cl2]在HT-1080细胞中诱导的细胞死亡机制中包含了铁突变。此外,我们还发现了 Prdx6 在调节 A-375 细胞对乌拉诺芬处理的敏感性方面的潜在作用,而本文评估的金(III)化合物则表现出不依赖于 Prdx6 的细胞毒性。最后,我们利用质谱分析表明,在体外酸性条件下,乌拉诺芬会选择性地与 Prdx6 的催化 Cys47 残基结合。与 C47S 突变体或在中性 pH 下均未观察到结合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


