Discovery of natural product derivative triptolidiol as a direct NLRP3 inhibitor by reducing K63-specific ubiquitination
Abstract
Background and Purpose
NLRP3 is up-regulated in inflammatory and autoimmune diseases. The development of NLRP3 inhibitors is challenged by the identification of compounds with distinct mechanisms of action avoiding side effects and toxicity. Triptolide is a natural product with multiple anti-inflammatory activities, but a narrow therapeutic window.
Experimental Approach
Natural product triptolide derivatives were screened for NLRP3 inhibitors in human THP-1 and mouse bone marrow-derived macrophages. The efficacy of potent NLRP3 inhibitors was evaluated in LPS-induced acute lung injury and septic shock models.
Key Results
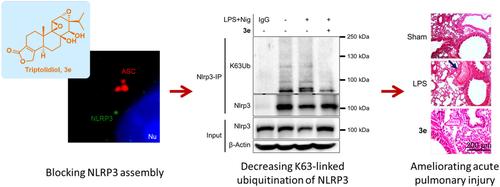
Triptolidiol was identified as a selective inhibitor of NLRP3 with high potency. Triptolidiol inactivated the NLRP3 inflammasome in human THP-1 and mouse primary macrophages primed with LPS. Triptolidiol specifically inhibited pro-caspase 1 cleavage downstream of NLRP3, but not AIM2 or NLRC4 inflammasomes. Based on the structure–activity relationship study, the C8-β-OH group was critical for its binding to NLRP3. Triptolidiol exhibited a submicromolar KD for NLRP3, binding to residue C280. This binding prevented the interaction of NLRP3 with NEK7, the key regulator of NLRP3 inflammasome oligomerization and assembly, but not with the inflammasome adaptor protein ASC. Triptolidiol decreased the K63-specific ubiquitination of NLRP3, leading NLRP3 to a “closed” inactive conformation. Intraperitoneal administration of triptolidiol significantly attenuated LPS-induced acute lung injury and lethal septic shock.
Conclusion and Implications
Triptolidiol is a novel NLRP3 inhibitor that regulates inflammasome assembly and activation by decreasing K63-linked ubiquitination. Triptolidiol has novel structural features that make it distinct from reported NLRP3 inhibitors and represents a viable therapeutic lead for inflammatory diseases.
LINKED ARTICLES
This article is part of a themed issue Drugs and Drug Targets in Metabolic and Chronic Inflammatory Diseases. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v182.20/issuetoc





 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


