LUBAC enables tumor-promoting LTβ receptor signaling by activating canonical NF-κB
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Lymphotoxin β receptor (LTβR), a member of the TNF receptor superfamily (TNFR-SF), is essential for development and maturation of lymphoid organs. In addition, LTβR activation promotes carcinogenesis by inducing a proinflammatory secretome. Yet, we currently lack a detailed understanding of LTβR signaling. In this study we discovered the linear ubiquitin chain assembly complex (LUBAC) as a previously unrecognized and functionally crucial component of the native LTβR signaling complex (LTβR-SC). Mechanistically, LUBAC-generated linear ubiquitin chains enable recruitment of NEMO, OPTN and A20 to the LTβR-SC, where they act coordinately to regulate the balance between canonical and non-canonical NF-κB pathways. Thus, different from death receptor signaling, where LUBAC prevents inflammation through inhibition of cell death, in LTβR signaling LUBAC is required for inflammatory signaling by enabling canonical and interfering with non-canonical NF-κB activation. This results in a LUBAC-dependent LTβR-driven inflammatory, protumorigenic secretome. Intriguingly, in liver cancer patients with high LTβR expression, high expression of LUBAC correlates with poor prognosis, providing clinical relevance for LUBAC-mediated inflammatory LTβR signaling.
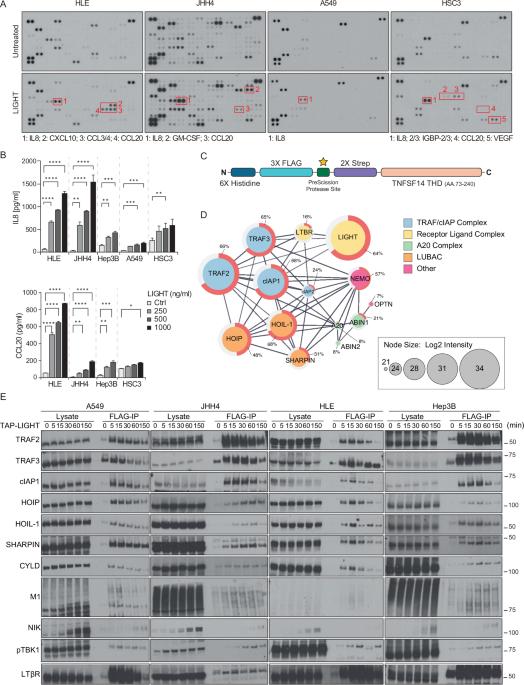
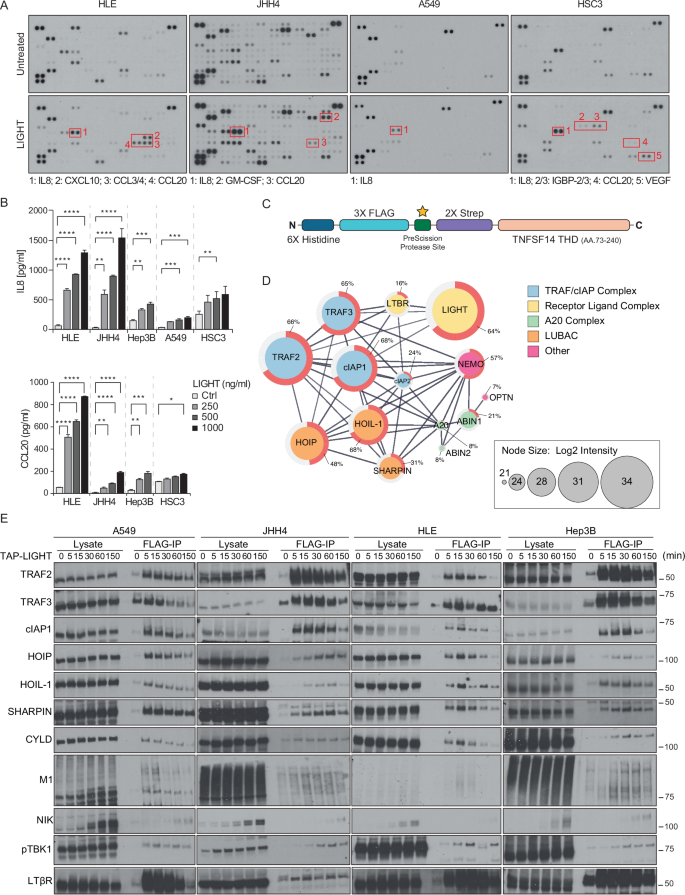
LUBAC 通过激活典型的 NF-κB 来实现促进肿瘤的 LTβ 受体信号转导
淋巴毒素β受体(LTβR)是 TNF 受体超家族(TNFR-SF)的成员,对淋巴器官的发育和成熟至关重要。此外,LTβR 的活化还能通过诱导促炎分泌组来促进癌变。然而,我们目前还缺乏对 LTβR 信号转导的详细了解。在这项研究中,我们发现了线性泛素链组装复合物(LUBAC),它是以前未曾认识到的原生 LTβR 信号传导复合物(LTβR-SC)的一个重要功能成分。从机理上讲,LUBAC 生成的线性泛素链能将 NEMO、OPTN 和 A20 招募到 LTβR-SC,它们在 LTβR-SC 中协调作用,调节规范和非规范 NF-κB 通路之间的平衡。因此,与死亡受体信号不同的是,LUBAC 通过抑制细胞死亡来防止炎症,而在 LTβR 信号中,LUBAC 是炎症信号所必需的,它可以激活规范 NF-κB 并干扰非规范 NF-κB 的激活。这就形成了依赖于 LUBAC 的 LTβR 驱动的炎性原癌基因分泌组。耐人寻味的是,在LTβR高表达的肝癌患者中,LUBAC的高表达与预后不良相关,这为LUBAC介导的LTβR炎症信号转导提供了临床意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


