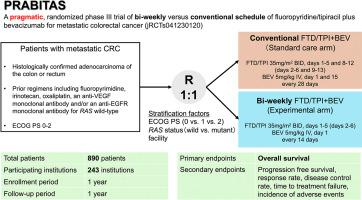
PRABITAS study design: a pragmatic, randomized phase III trial of bi-weekly versus conventional trifluridine/tipiracil plus bevacizumab for metastatic colorectal cancer
Abstract
Trifluridine/tipiracil (FTD/TPI) plus bevacizumab (BEV) is the established therapy for refractory metastatic colorectal cancer, but there are concerns regarding the regimen’s complexity and hematotoxic effects, especially for patients with organ dysfunction, comorbidities, or a reduced performance status—groups often excluded from conventional clinical trials. Preliminary studies demonstrated that bi-weekly FTD/TPI + BEV may mitigate these hematotoxic effects compared with the conventional schedule without compromising efficacy. No clinical trials, however, have directly compared these two regimens. Therefore, we initiated the PRABITAS trial, a multicenter, randomized, phase III non-inferiority trial, to evaluate the efficacy and safety of bi-weekly FTD/TPI + BEV compared with conventional FTD/TPI + BEV. This was designed as a pragmatic trial, a novel approach in clinical trials aiming to aid decision-making in daily practice by mimicking real-world clinical settings. The PRABITAS trial incorporates minimal eligibility criteria to include a more representative patient population, allows flexibility in intervention adherence and assessment, and employs streamlined data collection to reduce the burden on both patients and healthcare providers. The primary endpoint is overall survival in the intention-to-treat population. Launched in December 2023, the trial aimed to enroll a total of 890 patients.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


