Synthesis and anti-ureolitic activity of Biginelli adducts derived from formylphenyl boronic acids
Abstract
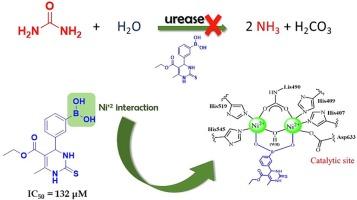
Urease is a metalloenzyme that contains two Ni(II) ions in its active site and catalyzes the hydrolysis of urea into ammonia and carbon dioxide. The development of effective urease inhibitors is crucial not only for mitigating nitrogen losses in agriculture but also for offering an alternative treatment against infections caused by resistant pathogens that utilize urease as a virulence factor. This study focuses on synthesizing and investigating the urease inhibition potential of Biginelli Adducts bearing a boric acid group. An unsubstituted or hydroxy-substituted boronic group in the Biginelli adducts structure enhances the urease inhibitory activity. Biophysical and kinetics studies revealed that the best Biginelli adduct (4e; IC50 = 132 ± 12 µmol/L) is a mixed inhibitor with higher affinity to the urease active site over an allosteric one. Docking studies confirm the interactions of 4e with residues essential for urease activity and demonstrate its potential to coordinate with the nickel atoms through the oxygen atoms of carbonyl or boronic acid groups. Overall, the Biginelli adduct 4e shows great potential as an additive for developing enhanced efficiency fertilizers and/or for medical applications.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


