Mapping the regulatory landscape for artificial intelligence in health within the European Union
IF 12.4
1区 医学
Q1 HEALTH CARE SCIENCES & SERVICES
引用次数: 0
Abstract
Regulatory frameworks for artificial intelligence (AI) are needed to mitigate risks while ensuring the ethical, secure, and effective implementation of AI technology in healthcare and population health. In this article, we present a synthesis of 141 binding policies applicable to AI in healthcare and population health in the EU and 10 European countries. The EU AI Act sets the overall regulatory framework for AI, while other legislations set social, health, and human rights standards, address the safety of technologies and the implementation of innovation, and ensure the protection and safe use of data. Regulation specifically pertaining to AI is still nascent and scarce, though a combination of data, technology, innovation, and health and human rights policy has already formed a baseline regulatory framework for AI in health. Future work should explore specific regulatory challenges, especially with respect to AI medical devices, data protection, and data enablement.
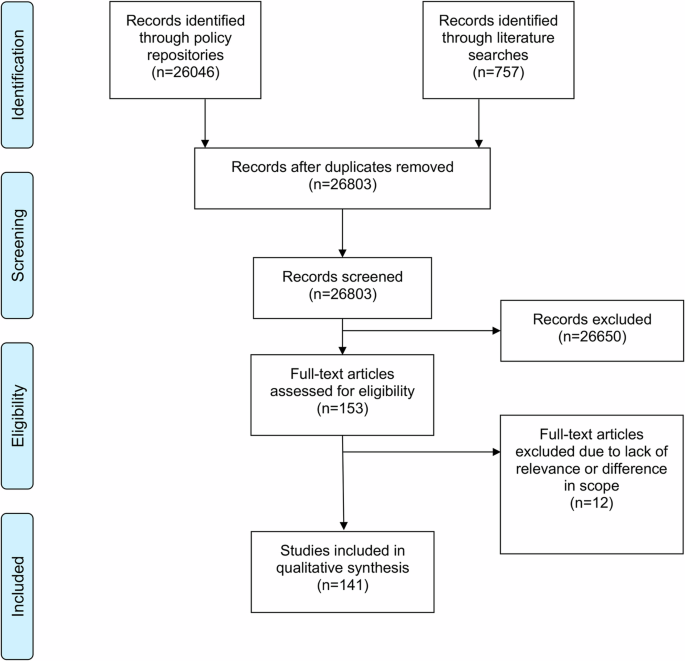
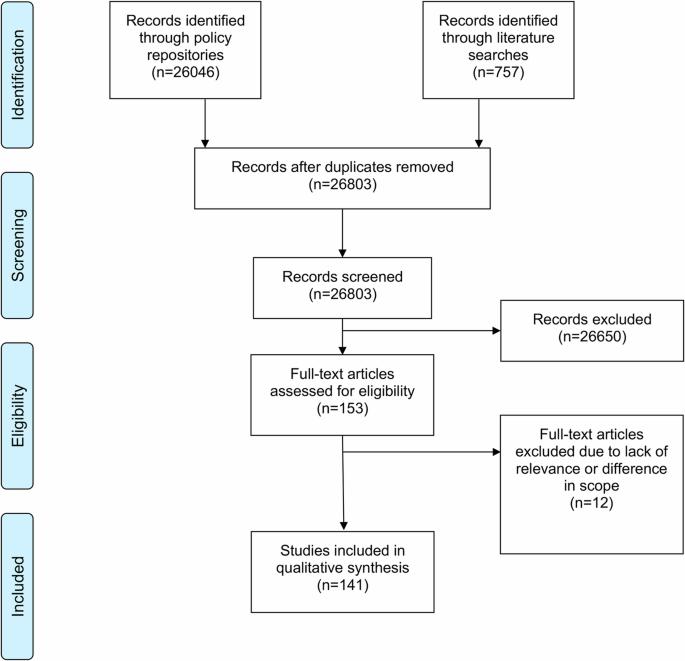
绘制欧盟内人工智能在健康领域的监管图景。
需要制定人工智能(AI)监管框架,以降低风险,同时确保在医疗保健和人口健康领域合乎道德、安全和有效地实施人工智能技术。在本文中,我们综合介绍了欧盟和 10 个欧洲国家适用于医疗保健和人口健康领域人工智能的 141 项具有约束力的政策。欧盟《人工智能法》为人工智能制定了总体监管框架,而其他立法则制定了社会、健康和人权标准,涉及技术安全和创新的实施,并确保数据的保护和安全使用。虽然数据、技术、创新以及健康和人权政策的结合已经形成了人工智能在健康领域的基准监管框架,但专门针对人工智能的法规仍处于起步阶段,且数量稀少。未来的工作应探索具体的监管挑战,特别是在人工智能医疗设备、数据保护和数据启用方面。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

NPJ Digital Medicine
Multiple-
CiteScore
25.10
自引率
3.30%
发文量
170
审稿时长
15 weeks
期刊介绍:
npj Digital Medicine is an online open-access journal that focuses on publishing peer-reviewed research in the field of digital medicine. The journal covers various aspects of digital medicine, including the application and implementation of digital and mobile technologies in clinical settings, virtual healthcare, and the use of artificial intelligence and informatics.
The primary goal of the journal is to support innovation and the advancement of healthcare through the integration of new digital and mobile technologies. When determining if a manuscript is suitable for publication, the journal considers four important criteria: novelty, clinical relevance, scientific rigor, and digital innovation.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


