Third-generation anti-CD19 CAR T cells for relapsed/refractory chronic lymphocytic leukemia: a phase 1/2 study
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
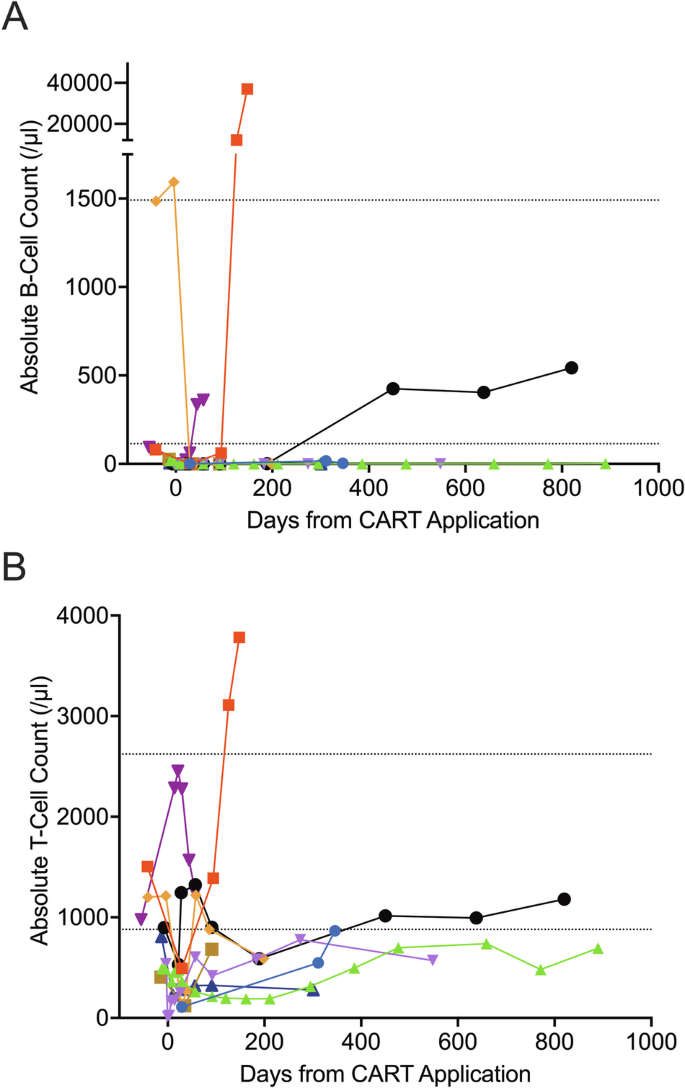
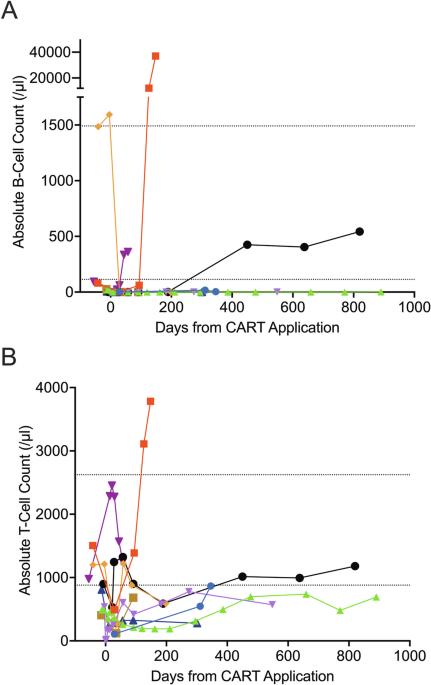
Third-generation chimeric antigen receptor T cells (CARTs) for relapsed or refractory (r/r) chronic lymphocytic leukemia (CLL) may improve efficacy compared to second-generation CARTs due to their enhanced CAR design. We performed the first phase 1/2 investigator-initiated trial evaluating escalating doses of third-generation CARTs (HD-CAR-1) targeting CD19 in patients with r/r CLL and B-cell lymphoma. CLL eligibility criteria were failure to two therapy lines including at least one pathway inhibitor and/or allogeneic hematopoietic cell transplantation. Nine heavily pretreated patients received HD-CAR-1 at dose levels ranging from 1 × 106 to 200 × 106 CART/m2. In-house HD-CAR-1 manufacturing was successful for all patients. While neurotoxicity was absent, one case of grade 3 cytokine release syndrome was observed. By day 90, six patients (67%) attained a CR, five of these (83%) with undetectable MRD. With a median follow-up of 27 months, 2-year PFS and OS were 30% and 69%, respectively. HD-CAR-1 products of responders contained significantly more CD4 + T cells compared to non-responders. In non-responders, a strong enrichment of effector memory-like CD8 + T cells with high expression of CD39 and/or CD197 was observed. HD-CAR-1 demonstrated encouraging efficacy and exceptionally low treatment-specific toxicity, presenting new treatment options for patients with r/r CLL. Trial registration: #NCT03676504.
第三代抗 CD19 CAR T 细胞治疗复发/难治性慢性淋巴细胞白血病:1/2 期研究。
第三代嵌合抗原受体 T 细胞(CART)用于治疗复发或难治性(r/r)慢性淋巴细胞白血病(CLL)的疗效可能会比第二代 CART 更好,因为其增强了 CAR 设计。我们进行了首例由研究者发起的1/2期试验,评估了针对r/r CLL和B细胞淋巴瘤患者的CD19第三代CARTs(HD-CAR-1)的递增剂量。CLL的入选标准是两种疗法失败,包括至少一种途径抑制剂和/或异体造血细胞移植。九名重度预处理患者接受了剂量为 1 × 106 至 200 × 106 CART/m2 的 HD-CAR-1。所有患者的 HD-CAR-1 均在公司内部成功生产。虽然不存在神经毒性,但观察到一例 3 级细胞因子释放综合征。到第 90 天,六名患者(67%)达到 CR,其中五名(83%)检测不到 MRD。中位随访时间为 27 个月,2 年的 PFS 和 OS 分别为 30% 和 69%。与非应答者相比,应答者的HD-CAR-1产物含有明显更多的CD4 + T细胞。在非应答者中,观察到高表达 CD39 和/或 CD197 的效应记忆型 CD8 + T 细胞大量富集。HD-CAR-1显示出令人鼓舞的疗效和极低的治疗特异性毒性,为r/r CLL患者提供了新的治疗选择。试验注册:#NCT03676504。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


