A multidimensional analysis reveals distinct immune phenotypes and the composition of immune aggregates in pediatric acute myeloid leukemia
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
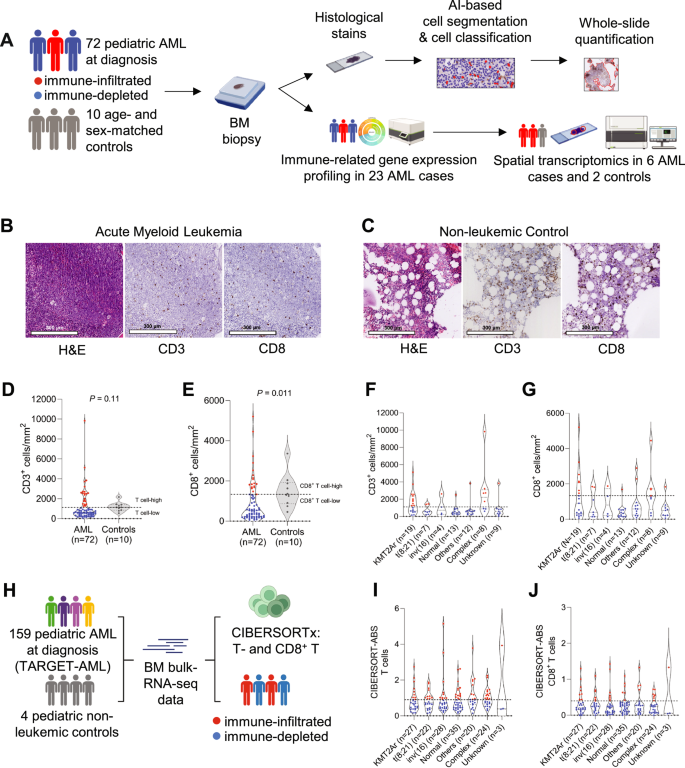
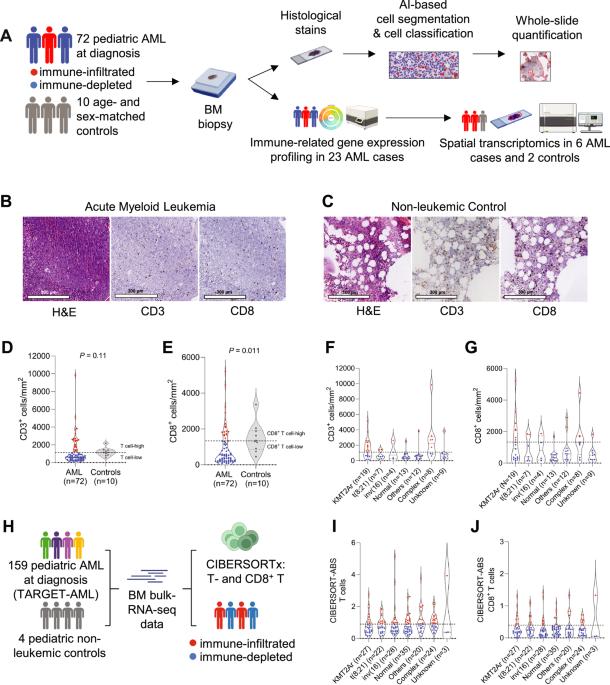
Because of the low mutational burden and consequently, fewer potential neoantigens, children with acute myeloid leukemia (AML) are thought to have a T cell-depleted or ‘cold’ tumor microenvironment and may have a low likelihood of response to T cell-directed immunotherapies. Understanding the composition, phenotype, and spatial organization of T cells and other microenvironmental populations in the pediatric AML bone marrow (BM) is essential for informing future immunotherapeutic trials about targetable immune-evasion mechanisms specific to pediatric AML. Here, we conducted a multidimensional analysis of the tumor immune microenvironment in pediatric AML and non-leukemic controls. We demonstrated that nearly one-third of pediatric AML cases has an immune-infiltrated BM, which is characterized by a decreased ratio of M2- to M1-like macrophages. Furthermore, we detected the presence of large T cell networks, both with and without colocalizing B cells, in the BM and dissected the cellular composition of T- and B cell-rich aggregates using spatial transcriptomics. These analyses revealed that these aggregates are hotspots of CD8+ T cells, memory B cells, plasma cells and/or plasmablasts, and M1-like macrophages. Collectively, our study provides a multidimensional characterization of the BM immune microenvironment in pediatric AML and indicates starting points for further investigations into immunomodulatory mechanisms in this devastating disease.
多维分析揭示了小儿急性髓性白血病中独特的免疫表型和免疫聚集体的组成。
由于突变负荷低,因此潜在的新抗原较少,急性髓性白血病(AML)患儿被认为具有T细胞贫乏或 "冷 "的肿瘤微环境,对T细胞导向的免疫疗法产生反应的可能性较低。了解小儿急性髓细胞性白血病骨髓(BM)中T细胞和其他微环境群体的组成、表型和空间组织对于未来的免疫治疗试验了解小儿急性髓细胞性白血病特有的可靶向免疫逃避机制至关重要。在这里,我们对小儿急性髓细胞白血病和非白血病对照组的肿瘤免疫微环境进行了多维分析。我们发现,近三分之一的小儿急性髓细胞性白血病病例具有免疫浸润性骨髓组织,其特征是 M2 型和 M1 型巨噬细胞的比例下降。此外,我们还检测到骨髓组织中存在大型 T 细胞网络,其中既有 B 细胞,也有不共聚焦的 B 细胞,并利用空间转录组学分析了富含 T 细胞和 B 细胞的聚集体的细胞组成。这些分析表明,这些聚集体是 CD8+ T 细胞、记忆性 B 细胞、浆细胞和/或浆细胞以及 M1 样巨噬细胞的热点。总之,我们的研究提供了小儿急性髓细胞白血病骨髓免疫微环境的多维特征,为进一步研究这种毁灭性疾病的免疫调节机制指明了起点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


