Fatty acid metabolism constrains Th9 cell differentiation and antitumor immunity via the modulation of retinoic acid receptor signaling
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
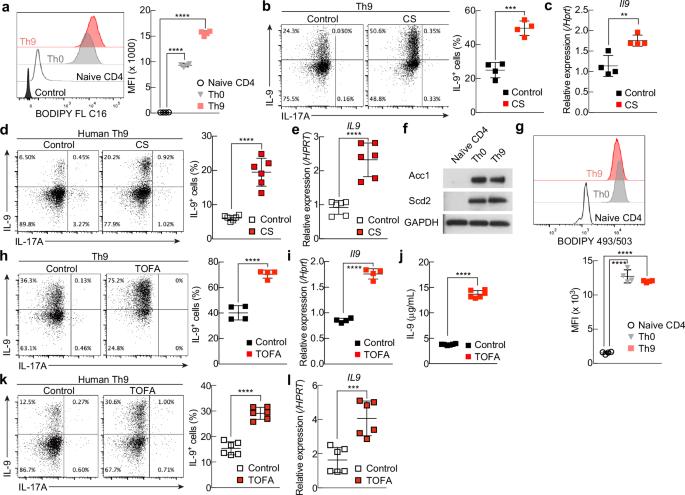
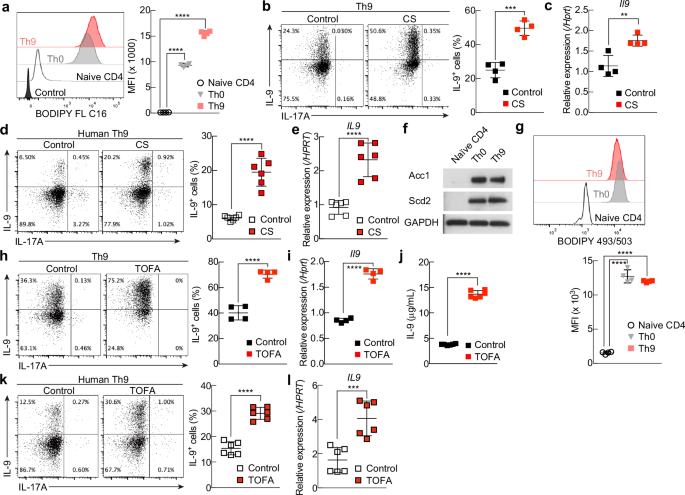
T helper 9 (Th9) cells are interleukin 9 (IL-9)-producing cells that have diverse functions ranging from antitumor immune responses to allergic inflammation. Th9 cells differentiate from naïve CD4+ T cells in the presence of IL-4 and transforming growth factor-beta (TGF-β); however, our understanding of the molecular basis of their differentiation remains incomplete. Previously, we reported that the differentiation of another subset of TGF-β–driven T helper cells, Th17 cells, is highly dependent on de novo lipid biosynthesis. On the basis of these findings, we hypothesized that lipid metabolism may also be important for Th9 cell differentiation. We therefore investigated the differentiation and function of mouse and human Th9 cells in vitro under conditions of pharmacologically or genetically induced deficiency of the intracellular fatty acid content and in vivo in mice genetically deficient in acetyl-CoA carboxylase 1 (ACC1), an important enzyme for fatty acid biosynthesis. Both the inhibition of de novo fatty acid biosynthesis and the deprivation of environmental lipids augmented differentiation and IL-9 production in mouse and human Th9 cells. Mechanistic studies revealed that the increase in Th9 cell differentiation was mediated by the retinoic acid receptor and the TGF-β–SMAD signaling pathways. Upon adoptive transfer, ACC1-inhibited Th9 cells suppressed tumor growth in murine models of melanoma and adenocarcinoma. Together, our findings highlight a novel role of fatty acid metabolism in controlling the differentiation and in vivo functions of Th9 cells.
脂肪酸代谢通过调节视黄酸受体信号制约 Th9 细胞分化和抗肿瘤免疫力
T辅助9(Th9)细胞是产生白细胞介素9(IL-9)的细胞,具有从抗肿瘤免疫反应到过敏性炎症等多种功能。Th9细胞在IL-4和转化生长因子-β(TGF-β)的作用下从幼稚CD4+ T细胞分化而来;然而,我们对其分化的分子基础的了解仍不全面。此前,我们曾报道,TGF-β驱动的T辅助细胞的另一个亚群--Th17细胞的分化高度依赖于新的脂质生物合成。基于这些发现,我们推测脂质代谢可能对 Th9 细胞的分化也很重要。因此,我们在体外研究了小鼠和人类 Th9 细胞在药物或基因诱导的细胞内脂肪酸含量缺乏条件下的分化和功能,并在体内研究了乙酰-CoA 羧化酶 1(ACC1)(一种脂肪酸生物合成的重要酶)基因缺乏的小鼠。抑制脂肪酸的新生物合成和剥夺环境中的脂质都会促进小鼠和人类 Th9 细胞的分化和 IL-9 的产生。机理研究显示,Th9细胞分化的增加是由维甲酸受体和TGF-β-SMAD信号通路介导的。在小鼠黑色素瘤和腺癌模型中,被ACC1抑制的Th9细胞经收养性转移后抑制了肿瘤的生长。总之,我们的研究结果凸显了脂肪酸代谢在控制 Th9 细胞分化和体内功能方面的新作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


