The co-location of CD14+APOE+ cells and MMP7+ tumour cells contributed to worse immunotherapy response in non-small cell lung cancer
Abstract
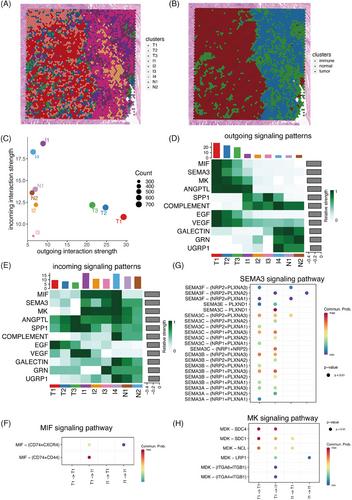
Intra-tumour immune infiltration is a crucial determinant affecting immunotherapy response in non-small cell lung cancer (NSCLC). However, its phenotype and related spatial structure have remained elusive. To overcome these restrictions, we undertook a comprehensive study comprising spatial transcriptomic (ST) data (28 712 spots from six samples). We identified two distinct intra-tumour infiltration patterns: immune exclusion (characterised by myeloid cells) and immune activation (characterised by plasma cells). The immune exclusion and immune activation signatures showed adverse and favourable roles in NSCLC patients' survival, respectively. Notably, CD14+APOE+ cells were recognised as the main cell type in immune exclusion samples, with increased epithelial‒mesenchymal transition and decreased immune activities. The co-location of CD14+APOE+ cells and MMP7+ tumour cells was observed in both ST and bulk transcriptomics data, validated by multiplex immunofluorescence performed on 20 NSCLC samples. The co-location area exhibited the upregulation of proliferation-related pathways and hypoxia activities. This co-localisation inhibited T-cell infiltration and the formation of tertiary lymphoid structures. Both CD14+APOE+ cells and MMP7+ tumour cells were associated with worse survival. In an immunotherapy cohort from the ORIENT-3 clinical trial, NSCLC patients who responded unfavourably exhibited higher infiltration of CD14+APOE+ cells and MMP7+ tumour cells. Within the co-location area, the MK, SEMA3 and Macrophage migration inhibitory factor (MIF) signalling pathway was most active in cell‒cell communication. This study identified immune exclusion and activation patterns in NSCLC and the co-location of CD14+APOE+ cells and MMP7+ tumour cells as contributors to immune resistance.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


