Quantifying Forms and Functions of Enterohepatic Bile Acid Pools in Mice
IF 7.1
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
Cellular and Molecular Gastroenterology and Hepatology
Pub Date : 2024-01-01
DOI:10.1016/j.jcmgh.2024.101392
引用次数: 0
Abstract
Backgrounds & Aims
Bile acids (BAs) are core gastrointestinal metabolites with dual functions in lipid absorption and cell signaling. BAs circulate between the liver and distal small intestine (i.e., ileum), yet the dynamics through which complex BA pools are absorbed in the ileum and interact with host intestinal cells in vivo remain poorly understood. Because ileal absorption is rate-limiting in determining which BAs in the intestinal lumen gain access to host intestinal cells and receptors, and at what concentrations, we hypothesized that defining the rates and routes of ileal BA absorption in vivo would yield novel insights into the physiological forms and functions of mouse enterohepatic BA pools.
Methods
Using ex vivo mass spectrometry, we quantified 88 BA species and metabolites in the intestinal lumen and superior mesenteric vein of individual wild-type mice, and cage-mates lacking the ileal BA transporter, Asbt/Slc10a2.
Results
Using these data, we calculated that the pool of BAs circulating through ileal tissue (i.e., the ileal BA pool) in fasting C57BL/6J female mice is ∼0.3 μmol/g. Asbt-mediated transport accounted for ∼80% of this pool and amplified size. Passive permeability explained the remaining ∼20% and generated diversity. Compared with wild-type mice, the ileal BA pool in Asbt-deficient mice was ∼5-fold smaller, enriched in secondary BA species and metabolites normally found in the colon, and elicited unique transcriptional responses on addition to ex vivo–cultured ileal explants.
Conclusions
This study defines quantitative traits of the mouse enterohepatic BA pool and reveals how aberrant BA metabolism can impinge directly on host intestinal physiology.
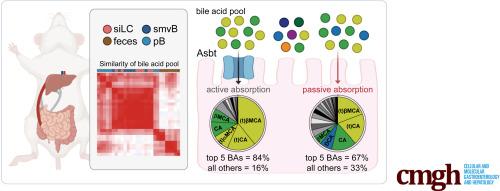
量化小鼠肠肝胆汁酸池的形式和功能。
背景与目的:胆汁酸(BA)是胃肠道的核心代谢产物,具有脂质吸收和细胞信号传导的双重功能。胆汁酸在肝脏和远端小肠(即回肠)之间循环,但复杂的胆汁酸池在回肠被吸收并在体内与宿主肠道细胞相互作用的动态过程仍鲜为人知。由于回肠吸收是决定肠腔中哪些 BA 能进入宿主肠细胞和受体以及浓度的速率限制,我们假设确定体内回肠吸收 BA 的速率和途径将能对小鼠肠肝 BA 池的生理形式和功能产生新的认识:我们使用体外质谱法对野生型小鼠和缺乏回肠BA转运体Asbt/Slc10a2的笼养小鼠肠腔和肠系膜上静脉中的88种BA和代谢物进行了定量分析:利用这些数据,我们计算出空腹的 C57BL/6J 雌性小鼠通过回肠组织循环的 BAs 池(即 "回肠 BA 池")为 ∼0.3 μmoles/g。Asbt介导的转运占到该库的80%,并扩大了其规模。被动渗透解释了剩余的 20%,并产生了多样性。与野生型小鼠相比,Asbt缺陷小鼠的回肠BA池小5倍,富含二级BA物种和通常在结肠中发现的代谢产物,并且在加入体内外培养的回肠外植体后会引起独特的转录反应:这项研究确定了小鼠肠肝 BA 库的定量特征,揭示了 BA 代谢异常如何直接影响宿主肠道生理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular and Molecular Gastroenterology and Hepatology
Medicine-Gastroenterology
CiteScore
13.00
自引率
2.80%
发文量
246
审稿时长
42 days
期刊介绍:
"Cell and Molecular Gastroenterology and Hepatology (CMGH)" is a journal dedicated to advancing the understanding of digestive biology through impactful research that spans the spectrum of normal gastrointestinal, hepatic, and pancreatic functions, as well as their pathologies. The journal's mission is to publish high-quality, hypothesis-driven studies that offer mechanistic novelty and are methodologically robust, covering a wide range of themes in gastroenterology, hepatology, and pancreatology.
CMGH reports on the latest scientific advances in cell biology, immunology, physiology, microbiology, genetics, and neurobiology related to gastrointestinal, hepatobiliary, and pancreatic health and disease. The research published in CMGH is designed to address significant questions in the field, utilizing a variety of experimental approaches, including in vitro models, patient-derived tissues or cells, and animal models. This multifaceted approach enables the journal to contribute to both fundamental discoveries and their translation into clinical applications, ultimately aiming to improve patient care and treatment outcomes in digestive health.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


