Mrgprb2-dependent Mast Cell Activation Plays a Crucial Role in Acute Colitis
IF 7.1
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
Cellular and Molecular Gastroenterology and Hepatology
Pub Date : 2024-01-01
DOI:10.1016/j.jcmgh.2024.101391
引用次数: 0
Abstract
Background & Aims
Mast cells (MCs) are typically found at mucosal surfaces, where their immunoglobulin E (IgE)-dependent activation plays a central role in allergic diseases. Over the past years, signaling through Mas-related G protein-coupled receptor b2 (Mrgprb2) in mice and MRGPRX2 in humans has gained a lot of interest as an alternative MC activation pathway with high therapeutic potential. The aim of this study was to explore the relevance of such IgE-independent, Mrgprb2-mediated signaling in colonic MCs in the healthy and acutely inflamed mouse colon.
Methods
Mrgprb2 expression and functionality was studied using a genetic labeling strategy combined with advanced microscopic imaging. Furthermore, Mrgprb2 knockout (Mrgprb2-/-) mice were used to determine the role of this pathway in a preclinical dextran sodium sulphate (DSS) colitis model.
Results
We found that Mrgprb2 acts as a novel MC degranulation pathway in a large subset of connective tissue MCs in the mouse distal colon. Acute DSS colitis induced a pronounced increase of Mrgprb2-expressing MCs, which were found in close association with Substance P-positive nerve fibers. Loss of Mrgprb2-mediated signaling impaired DSS-induced neutrophil influx and significantly impacted on acute colitis progression.
Conclusions
Our findings uncover a novel, IgE-independent MC degranulation pathway in the mouse colon that plays a central role in acute colitis pathophysiology, mainly by safeguarding acute colitis progression and severity in mice. This pseudo allergic, Mrgprb2-induced signaling is part of a hitherto unconsidered colonic neuro-immune pathway and might have significant potential for the further development of effective therapeutic treatment strategies for gastrointestinal disorders, such as ulcerative colitis.
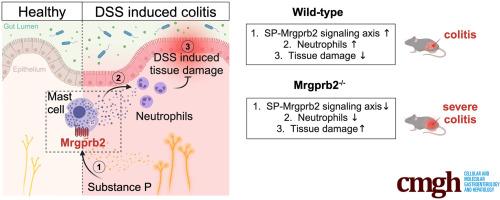
Mrgprb2依赖性肥大细胞活化在急性结肠炎中起着至关重要的作用。
背景与目的:肥大细胞(MC)通常存在于粘膜表面,其 IgE 依赖性活化在过敏性疾病中起着核心作用。在过去几年中,通过与 Mas 相关的 G 蛋白偶联受体 b2(Mrgprb2)(小鼠)和 MRGPRX2(人类)发出信号,作为一种具有高治疗潜力的替代 MC 激活途径,引起了人们的广泛兴趣。本研究的目的是探索这种不依赖于 IgE、由 Mrgprb2 介导的信号传导在健康和急性炎症小鼠结肠 MC 中的相关性:方法:采用基因标记策略结合先进的显微成像技术研究了Mrgprb2的表达和功能。此外,我们还利用 Mrgprb2 基因敲除(Mrgprb2-/-)小鼠来确定该通路在临床前右旋糖酐硫酸钠(DSS)结肠炎模型中的作用:结果:我们发现,Mrgprb2 在小鼠结肠远端结缔组织 MCs(CTMCs)的一个大型亚群中作为一种新型 MC 脱颗粒途径。急性DSS结肠炎诱导表达Mrgprb2的MCs明显增加,这些MCs与物质P(SP)阳性神经纤维密切相关。失去Mrgprb2介导的信号传导会影响DSS诱导的中性粒细胞流入,并显著影响急性结肠炎的进展:我们的研究结果揭示了小鼠结肠中一种新的、不依赖于 IgE 的 MC 脱颗粒途径,它在急性结肠炎病理生理学中发挥着核心作用,主要是通过保护小鼠急性结肠炎的进展和严重程度。这种由Mrgprb2诱导的假性过敏信号传导是迄今尚未被考虑的结肠神经免疫通路的一部分,可能对进一步开发有效的胃肠道疾病(如溃疡性结肠炎)治疗策略具有重大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular and Molecular Gastroenterology and Hepatology
Medicine-Gastroenterology
CiteScore
13.00
自引率
2.80%
发文量
246
审稿时长
42 days
期刊介绍:
"Cell and Molecular Gastroenterology and Hepatology (CMGH)" is a journal dedicated to advancing the understanding of digestive biology through impactful research that spans the spectrum of normal gastrointestinal, hepatic, and pancreatic functions, as well as their pathologies. The journal's mission is to publish high-quality, hypothesis-driven studies that offer mechanistic novelty and are methodologically robust, covering a wide range of themes in gastroenterology, hepatology, and pancreatology.
CMGH reports on the latest scientific advances in cell biology, immunology, physiology, microbiology, genetics, and neurobiology related to gastrointestinal, hepatobiliary, and pancreatic health and disease. The research published in CMGH is designed to address significant questions in the field, utilizing a variety of experimental approaches, including in vitro models, patient-derived tissues or cells, and animal models. This multifaceted approach enables the journal to contribute to both fundamental discoveries and their translation into clinical applications, ultimately aiming to improve patient care and treatment outcomes in digestive health.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


