Identification of KIFC1 as a putative vulnerability in lung cancers with centrosome amplification
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Centrosome amplification (CA), an abnormal increase in the number of centrosomes in the cell, is a recurrent phenomenon in lung and other malignancies. Although CA promotes tumor development and progression by inducing genomic instability (GIN), it also induces mitotic stress that jeopardizes cellular integrity. CA leads to the formation of multipolar mitotic spindles that can cause lethal chromosome segregation errors. To sustain the benefits of CA by mitigating its consequences, malignant cells are dependent on adaptive mechanisms that represent therapeutic vulnerabilities. We aimed to discover genetic dependencies associated with CA in lung cancer. Combining a CRISPR/Cas9 functional genomics screen with tumor genomic analyses, we identified the motor protein KIFC1, also known as HSET, as a putative vulnerability specifically in lung adenocarcinoma (LUAD) with CA. KIFC1 expression was positively correlated with CA in LUAD and associated with worse patient outcomes, smoking history, and indicators of GIN. KIFC1 loss-of-function sensitized LUAD cells with high basal KIFC1 expression to potentiation of CA, which was associated with a diminished ability to cluster extra centrosomes into pseudo-bipolar mitotic spindles. Our work suggests that KIFC1 inhibition represents a novel approach for potentiating GIN to lethal levels in LUAD with CA by forcing cells to divide with multipolar spindles, rationalizing further studies to investigate its therapeutic potential.
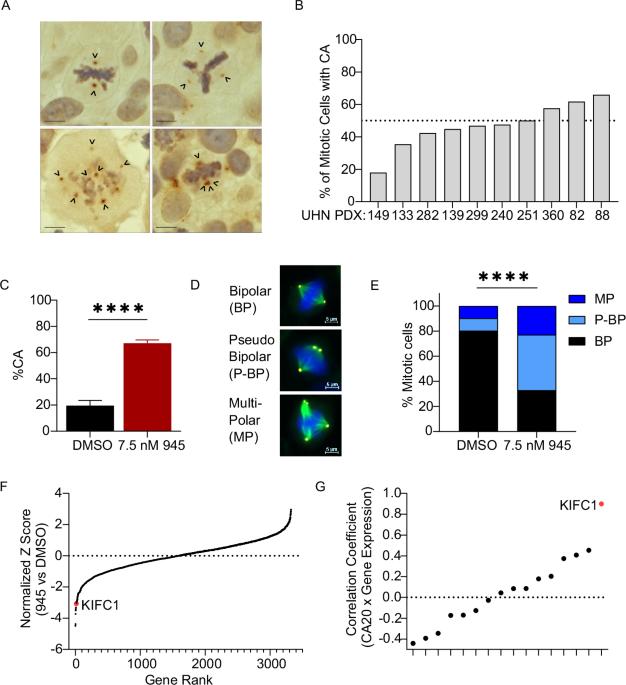

鉴定 KIFC1 在中心体扩增的肺癌中的潜在脆弱性。
中心体扩增(CA)是细胞中中心体数量的异常增加,是肺部和其他恶性肿瘤中经常出现的一种现象。尽管中心体扩增会诱发基因组不稳定性(GIN),从而促进肿瘤的发展和恶化,但它也会诱发有丝分裂压力,危及细胞的完整性。CA 会导致多极有丝分裂纺锤体的形成,从而造成致命的染色体分离错误。为了通过减轻 CA 的后果来维持 CA 的益处,恶性细胞依赖于代表治疗脆弱性的适应机制。我们旨在发现肺癌中与CA相关的遗传依赖性。结合 CRISPR/Cas9 功能基因组学筛选和肿瘤基因组分析,我们发现了运动蛋白 KIFC1(又称 HSET),它是肺腺癌(LUAD)与 CA 的特异性易感基因。KIFC1 的表达与 LUAD 中的 CA 呈正相关,并与患者的预后、吸烟史和 GIN 指标相关。KIFC1功能缺失会使基础KIFC1表达量高的LUAD细胞对CA的增效作用敏感,这与将额外的中心体聚集成假双极有丝分裂轴的能力减弱有关。我们的研究表明,KIFC1抑制是一种新方法,可通过迫使细胞以多极纺锤体方式分裂,将LUAD细胞中的GIN增效至致死水平,从而进一步研究其治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


