Gelsolin alleviates rheumatoid arthritis by negatively regulating NLRP3 inflammasome activation
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
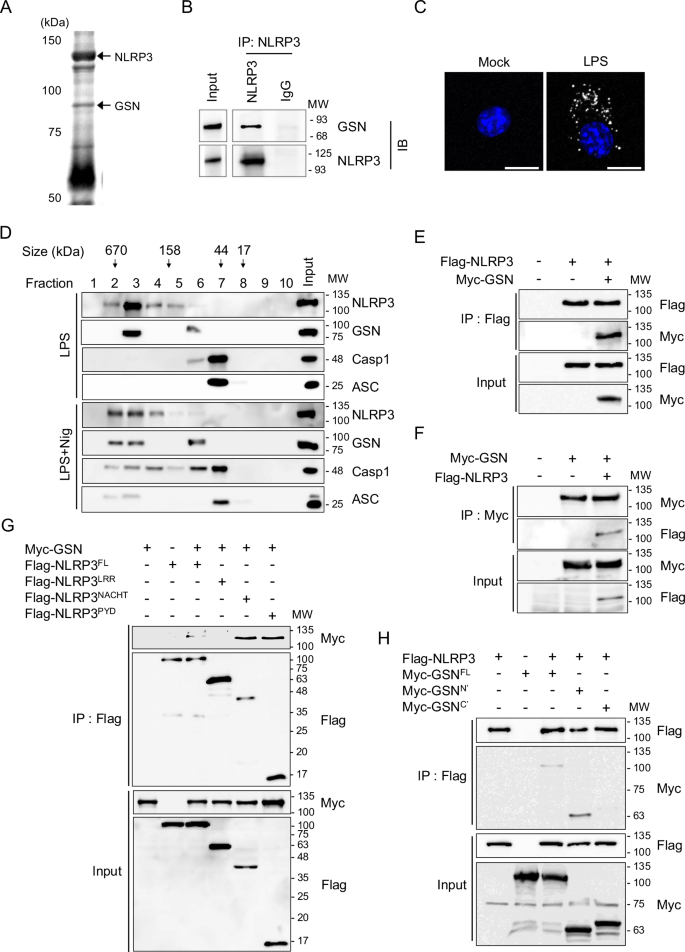
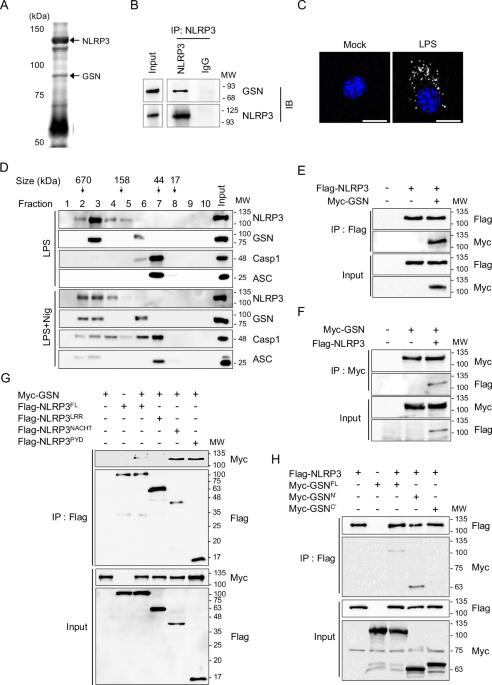
Despite numerous biomarkers being proposed for rheumatoid arthritis (RA), a gap remains in our understanding of their mechanisms of action. In this study, we discovered a novel role for gelsolin (GSN), an actin-binding protein whose levels are notably reduced in the plasma of RA patients. We elucidated that GSN is a key regulator of NLRP3 inflammasome activation in macrophages, providing a plausible explanation for the decreased secretion of GSN in RA patients. We found that GSN interacts with NLRP3 in LPS-primed macrophages, hence modulating the formation of the NLRP3 inflammasome complex. Reducing GSN expression significantly enhanced NLRP3 inflammasome activation. GSN impeded NLRP3 translocation to the mitochondria; it contributed to the maintenance of intracellular calcium equilibrium and mitochondrial stability. This maintenance is crucial for controlling the inflammatory response associated with RA. Furthermore, the exacerbation of arthritic symptoms in GSN-deficient mice indicates the potential of GSN as both a diagnostic biomarker and a therapeutic target. Moreover, not limited to RA models, GSN has demonstrated a protective function in diverse disease models associated with the NLRP3 inflammasome. Myeloid cell-specific GSN-knockout mice exhibited aggravated inflammatory responses in models of MSU-induced peritonitis, folic acid-induced acute tubular necrosis, and LPS-induced sepsis. These findings suggest novel therapeutic approaches that modulate GSN activity, offering promise for more effective management of RA and a broader spectrum of inflammatory conditions.
凝胶苷元通过负向调节 NLRP3 炎症小体的激活缓解类风湿性关节炎
尽管针对类风湿性关节炎(RA)提出了许多生物标志物,但我们对其作用机制的了解仍然存在差距。在这项研究中,我们发现了凝胶蛋白(GSN)的新作用,凝胶蛋白是一种肌动蛋白结合蛋白,在类风湿性关节炎患者血浆中的含量明显降低。我们阐明了GSN是巨噬细胞中NLRP3炎性体活化的关键调节因子,为RA患者GSN分泌减少提供了合理解释。我们发现,在LPS刺激的巨噬细胞中,GSN与NLRP3相互作用,从而调节NLRP3炎性体复合物的形成。减少GSN的表达会明显增强NLRP3炎性体的激活。GSN 阻碍了 NLRP3 向线粒体的转运;它有助于维持细胞内钙平衡和线粒体的稳定性。这种维持对于控制与 RA 相关的炎症反应至关重要。此外,GSN 缺陷小鼠关节炎症状的加重表明,GSN 具有作为诊断生物标志物和治疗靶点的潜力。此外,GSN不仅限于RA模型,还在与NLRP3炎性体相关的多种疾病模型中显示出保护功能。在MSU诱导的腹膜炎、叶酸诱导的急性肾小管坏死和LPS诱导的败血症模型中,髓系细胞特异性GSN基因敲除小鼠表现出加重的炎症反应。这些发现提出了调节 GSN 活性的新型治疗方法,有望更有效地治疗 RA 和更广泛的炎症。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


