Control physicochemical and electrochemical properties of Pr1.6Cа0.4Ni0.6Cu0.4O4+δ as a prospective cathode material for solid oxide cells through the synthesis process
Abstract
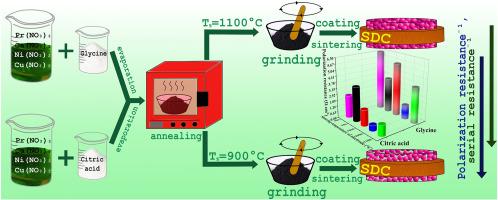
The aim of this work is to establish the relationship between the electrochemical performance of the Pr1.6Cа0.4Ni0.6Cu0.4O4+δ-based electrodes and the properties of the electrode powders, conditioned by their synthesis history, as well as the electrode design and the sintering conditions of the electrode layers. The Pr1.6Cа0.4Ni0.6Cu0.4O4+δ (PCNCO) powders are synthesized by combustion of salt compositions using different fuels: glycine, polyvinyl alcohol and citric acid. The influence of the composition of the redox mixture on the synthesis process, the phase composition of the obtained powders and their properties have been studied. The microstructure of the PCNCO electrodes formed from the powders with different dispersions is studied by electron microscopy. The electrochemical performance of the electrodes in contact with the Ce0.8Sm0.2O1.9 (SDC) electrolyte is studied by impedance spectroscopy. Based on the correlations established between the chemical stability and dispersion of the powders and the microstructure and polarization resistance of the corresponding electrodes, the optimal parameters for the synthesis of the PCNCO complex oxide for the use as a cathode material have been determined. The lowest polarization resistance equal to 0.38 Ω cm2 at 700 °C is obtained for the bilayer electrode with the PCNCO functional layer synthesized by the citrate-nitrate combustion and sintered at 1050 °C, and the LaNi0.6Fe0.4O3–δ oxide collector sintered at 900 °C. The developed synthesis procedure and electrode design can be recommended as promising for the fabrication of air electrodes in the intermediate-temperature electrochemical devices.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


