STING is crucial for the survival of RUNX1::RUNX1T1 leukemia cells
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
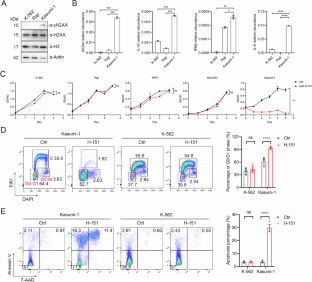
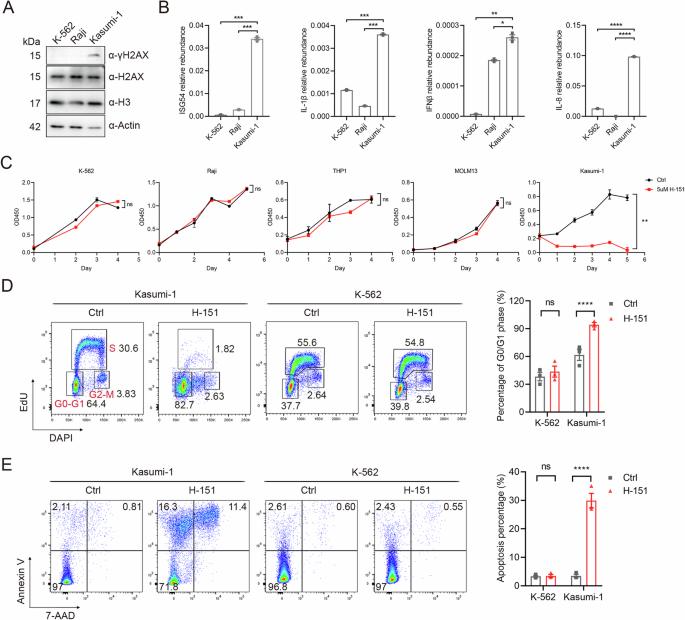
Even though acute myeloid leukemia (AML) patients with a RUNX1::RUNX1T1 (AE) fusion have a relatively favorable prognosis, approximately 50% relapse within 2.5 years and develop resistance to subsequent chemotherapy [1]. It is therefore imperative to identify novel therapeutic targets for AE leukemia to improve outcomes. In this study, we unveil that targeting STING effectively suppresses the growth of AE leukemia cells. Both genetic and pharmacological inhibition of STING lead to the diminish of AE leukemia cells. Importantly, in a mouse primary AE leukemia model, STING deletion significantly attenuates leukemogenesis and prolongs the animals’ lifespan. Blocking the downstream inflammatory pathway of STING yields similar effects to STING inhibition in AE leukemia cells, highlighting the pivotal role of STING-dependent inflammatory responses in sustaining the survival of AE leukemia cells. Moreover, through a genome-wide CRISPR screen, we identified fatty acid desaturase 2 (FADS2) as a non-canonical factor downstream of STING inhibition that mediates cell death. Inhibition of STING releases FADS2 activity, consequently inducing the synthesis of polyunsaturated fatty acids (PUFAs) and triggering lipid peroxidation-associated cell death [2]. Taken together, these findings reveal a critical function of STING in the survival of AE-positive AML cells and suggest STING to be a potential therapeutic target for clinical intervention in these patients.
STING 对 RUNX1::RUNX1T1 白血病细胞的存活至关重要
尽管RUNX1::RUNX1T1(AE)融合的急性髓性白血病(AML)患者预后相对较好,但约有50%的患者会在2.5年内复发,并对后续化疗产生抗药性[1]。因此,当务之急是找到AE白血病的新治疗靶点,以改善预后。在这项研究中,我们发现靶向 STING 能有效抑制 AE 白血病细胞的生长。遗传和药物抑制 STING 都会导致 AE 白血病细胞的减少。重要的是,在小鼠原发性AE白血病模型中,STING缺失能显著减轻白血病的发生并延长动物的寿命。阻断 STING 的下游炎症通路在 AE 白血病细胞中产生与 STING 抑制相似的效果,这凸显了 STING 依赖性炎症反应在维持 AE 白血病细胞存活中的关键作用。此外,通过全基因组CRISPR筛选,我们发现脂肪酸去饱和酶2(FADS2)是STING抑制下游介导细胞死亡的非经典因子。抑制 STING 会释放 FADS2 的活性,从而诱导多不饱和脂肪酸(PUFA)的合成,引发脂质过氧化相关的细胞死亡 [2]。综上所述,这些发现揭示了 STING 在 AE 阳性 AML 细胞存活过程中的关键功能,并提示 STING 是临床干预这些患者的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


