Identification of a novel alternative splicing isoform of the Hippo kinase STK3/MST2 with impaired kinase and cell growth suppressing activities
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
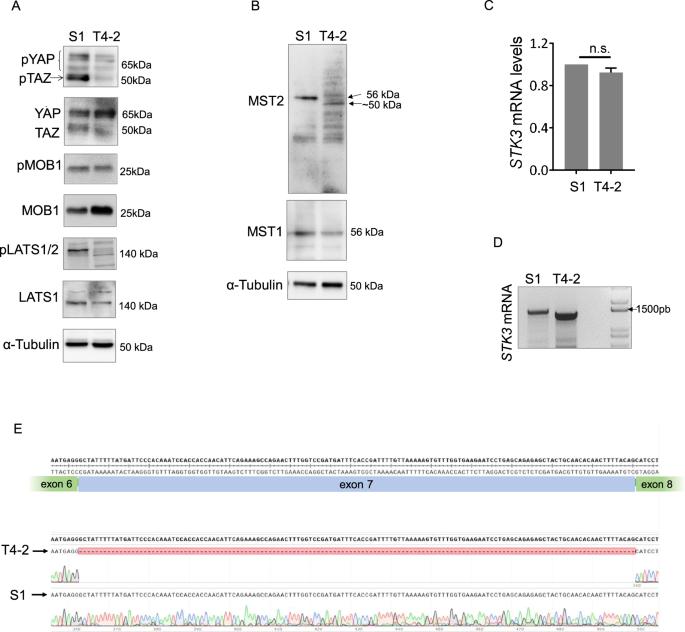
Mammalian Ste-20-like Kinases 1 and 2 (MST1/2) are core serine-threonine kinases of the Hippo pathway regulating several cellular processes, including cell cycle arrest and cell death. Here, we discovered a novel alternative splicing variant of the MST2 encoding gene, STK3, in malignant cells and tumor datasets. This variant, named STK3∆7 or MST2∆7 (for mRNA or protein, respectively), resulted from the skipping of exon 7. MST2∆7 exhibited increased ubiquitylation and interaction with the E3 ubiquitin-protein ligase CHIP compared to the full-length protein (MST2FL). Exon 7 in STK3 encodes a segment within the kinase domain, and its exclusion compromised MST2 interaction with and phosphorylation of MOB, a major MST1/2 substrate. Nevertheless, MST2∆7 was capable of interacting with MST1 and MST2FL. Unlike MST2FL, overexpression of MST2∆7 did not lead to increased cell death and growth arrest. Strikingly, we observed the exclusion of STK3 exon 7 in 3.2–15% of tumor samples from patients of several types of cancer, while STK3∆7 was seldomly found in healthy tissues. Our study identified a novel STK3 splicing variant with loss of function and the potential to disturb tissue homeostasis by impacting on MST2 activities in the regulation of cell death and quiescence.
鉴定出一种新的河马激酶 STK3/MST2 替代剪接异构体,其激酶和细胞生长抑制活性受损。
哺乳动物Ste-20样激酶1和2(MST1/2)是Hippo通路的核心丝氨酸-苏氨酸激酶,调节多种细胞过程,包括细胞周期停滞和细胞死亡。在这里,我们在恶性细胞和肿瘤数据集中发现了MST2编码基因STK3的一种新型替代剪接变体。这种变体被命名为 STK3∆7 或 MST2∆7 (分别表示 mRNA 或蛋白质),是由于跳过了第 7 号外显子。与全长蛋白(MST2FL)相比,MST2∆7 的泛素化程度和与 E3 泛素蛋白连接酶 CHIP 的相互作用都有所提高。STK3 的第 7 号外显子编码激酶结构域内的一个片段,排除该片段会影响 MST2 与 MOB(MST1/2 的主要底物)的相互作用和磷酸化。然而,MST2∆7 能够与 MST1 和 MST2FL 相互作用。与 MST2FL 不同,过量表达 MST2∆7 不会导致细胞死亡和生长停滞。令人震惊的是,我们观察到在几种癌症患者的 3.2-15% 的肿瘤样本中排除了 STK3 第 7 外显子,而在健康组织中很少发现 STK3∆7。我们的研究发现了一种新的 STK3 剪接变体,这种变体具有功能缺失的特性,有可能通过影响 MST2 在调节细胞死亡和静止过程中的活性来扰乱组织的平衡。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


