Single-cell transcriptomics identifies aberrant glomerular angiogenic signalling in the early stages of WT1 kidney disease
IF 5.6
2区 医学
Q1 ONCOLOGY
Jennifer C Chandler, Daniyal J Jafree, Saif Malik, Gideon Pomeranz, Mary Ball, Maria Kolatsi-Joannou, Alice Piapi, William J Mason, Andrew V Benest, David O Bates, Aleksandra Letunovska, Reem Al-Saadi, Marion Rabant, Olivia Boyer, Kathy Pritchard-Jones, Paul J Winyard, Andrew S Mason, Adrian S Woolf, Aoife M Waters, David A Long
下载PDF
{"title":"Single-cell transcriptomics identifies aberrant glomerular angiogenic signalling in the early stages of WT1 kidney disease","authors":"Jennifer C Chandler, Daniyal J Jafree, Saif Malik, Gideon Pomeranz, Mary Ball, Maria Kolatsi-Joannou, Alice Piapi, William J Mason, Andrew V Benest, David O Bates, Aleksandra Letunovska, Reem Al-Saadi, Marion Rabant, Olivia Boyer, Kathy Pritchard-Jones, Paul J Winyard, Andrew S Mason, Adrian S Woolf, Aoife M Waters, David A Long","doi":"10.1002/path.6339","DOIUrl":null,"url":null,"abstract":"<p><i>WT1</i> encodes a podocyte transcription factor whose variants can cause an untreatable glomerular disease in early childhood. Although WT1 regulates many podocyte genes, it is poorly understood which of them are initiators in disease and how they subsequently influence other cell-types in the glomerulus. We hypothesised that this could be resolved using single-cell RNA sequencing (scRNA-seq) and ligand-receptor analysis to profile glomerular cell–cell communication during the early stages of disease in mice harbouring an orthologous human mutation in WT1 (<i>Wt1</i><sup><i>R394W/+</i></sup>). Podocytes were the most dysregulated cell-type in the early stages of <i>Wt1</i><sup><i>R394W/+</i></sup> disease, with disrupted angiogenic signalling between podocytes and the endothelium, including the significant downregulation of transcripts for the vascular factors Vegfa and Nrp1. These signalling changes preceded glomerular endothelial cell loss in advancing disease, a feature also observed in biopsy samples from human WT1 glomerulopathies. Addition of conditioned medium from murine <i>Wt1</i><sup><i>R394W/+</i></sup> primary podocytes to wild-type glomerular endothelial cells resulted in impaired endothelial looping and reduced vascular complexity. Despite the loss of key angiogenic molecules in <i>Wt1</i><sup><i>R394W/+</i></sup> podocytes, the pro-vascular molecule adrenomedullin was upregulated in <i>Wt1</i><sup><i>R394W/+</i></sup> podocytes and plasma and its further administration was able to rescue the impaired looping observed when glomerular endothelium was exposed to <i>Wt1</i><sup><i>R394W/+</i></sup> podocyte medium. In comparative analyses, adrenomedullin upregulation was part of a common injury signature across multiple murine and human glomerular disease datasets, whilst other gene changes were unique to WT1 disease. Collectively, our study describes a novel role for altered angiogenic signalling in the initiation of WT1 glomerulopathy. We also identify adrenomedullin as a proangiogenic factor, which despite being upregulated in early injury, offers an insufficient protective response due to the wider milieu of dampened vascular signalling that results in endothelial cell loss in later disease. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 2","pages":"212-227"},"PeriodicalIF":5.6000,"publicationDate":"2024-08-23","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6339","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6339","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
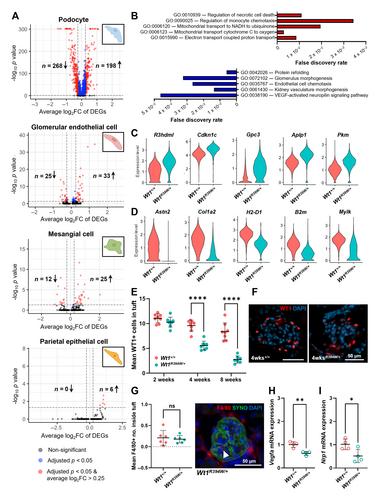
WT1 encodes a podocyte transcription factor whose variants can cause an untreatable glomerular disease in early childhood. Although WT1 regulates many podocyte genes, it is poorly understood which of them are initiators in disease and how they subsequently influence other cell-types in the glomerulus. We hypothesised that this could be resolved using single-cell RNA sequencing (scRNA-seq) and ligand-receptor analysis to profile glomerular cell–cell communication during the early stages of disease in mice harbouring an orthologous human mutation in WT1 (Wt1 R394W/+ Wt1 R394W/+ Wt1 R394W/+ Wt1 R394W/+ Wt1 R394W/+ Wt1 R394W/+ The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
单细胞转录组学发现 WT1 肾病早期肾小球血管生成信号异常。
WT1 是一种荚膜细胞转录因子,其变体可在儿童早期导致无法治疗的肾小球疾病。虽然 WT1 可调控许多荚膜细胞基因,但人们对其中哪些基因是疾病的始作俑者以及它们随后如何影响肾小球中的其他细胞类型还知之甚少。我们假设可以利用单细胞 RNA 测序(scRNA-seq)和配体受体分析来解决这一问题,即在携带人类 WT1 同源突变(Wt1R394W/+)的小鼠疾病早期阶段,分析肾小球细胞与细胞之间的交流。在 Wt1R394W/+ 疾病的早期阶段,荚膜细胞是失调最严重的细胞类型,荚膜细胞和内皮细胞之间的血管生成信号被破坏,包括血管因子 Vegfa 和 Nrp1 的转录物显著下调。这些信号变化先于肾小球内皮细胞的丧失而出现,在人类 WT1 肾小球疾病的活检样本中也观察到了这一特征。在野生型肾小球内皮细胞中加入小鼠 Wt1R394W/+ 原始荚膜细胞的条件培养基会导致内皮环路受损和血管复杂性降低。尽管 Wt1R394W/+ 荚膜细胞中丧失了关键的血管生成分子,但促血管生成分子肾上腺髓质素在 Wt1R394W/+ 荚膜细胞和血浆中上调,进一步施用肾上腺髓质素能挽救肾小球内皮暴露于 Wt1R394W/+ 荚膜细胞培养基时观察到的循环受损。在比较分析中,肾上腺髓质素上调是多个小鼠和人类肾小球疾病数据集中共同损伤特征的一部分,而其他基因变化则是 WT1 疾病所特有的。总之,我们的研究描述了血管生成信号的改变在 WT1 肾小球病变中的新作用。我们还发现肾上腺髓质素是一种促血管生成因子,尽管它在早期损伤时上调,但由于血管信号受抑制的环境更广泛,它不能提供足够的保护性反应,从而导致后期疾病中内皮细胞的丧失。© 2024 作者。病理学杂志》由约翰威利父子有限公司代表大不列颠及爱尔兰病理学会出版。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
来源期刊
期刊介绍:
The Journal of Pathology aims to serve as a translational bridge between basic biomedical science and clinical medicine with particular emphasis on, but not restricted to, tissue based studies. The main interests of the Journal lie in publishing studies that further our understanding the pathophysiological and pathogenetic mechanisms of human disease.
The Journal of Pathology welcomes investigative studies on human tissues, in vitro and in vivo experimental studies, and investigations based on animal models with a clear relevance to human disease, including transgenic systems.
As well as original research papers, the Journal seeks to provide rapid publication in a variety of other formats, including editorials, review articles, commentaries and perspectives and other features, both contributed and solicited.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


