Single-nucleus and spatial transcriptome reveal adrenal homeostasis in normal and tumoural adrenal glands
Abstract
The human adrenal gland is a complex endocrine tissue. Studies on adrenal renewal have been limited to animal models or human foetuses. Enhancing our understanding of adult human adrenal homeostasis is crucial for gaining insights into the pathogenesis of adrenal diseases, such as adrenocortical tumours.
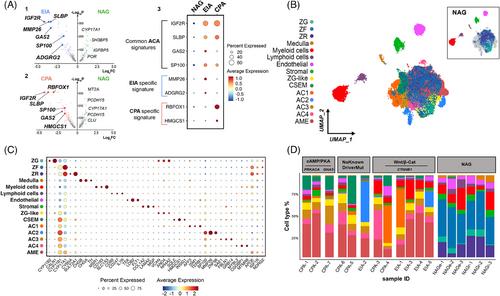
Here, we present a comprehensive cellular genomics analysis of the adult human normal adrenal gland, combining single-nuclei RNA sequencing and spatial transcriptome data to reconstruct adrenal gland homeostasis. As expected, we identified primary cells of the various zones of the adrenal cortex and medulla, but we also uncovered additional cell types. They constitute the adrenal microenvironment, including immune cells, mostly composed of a large population of M2 macrophages, and new cell populations, including different subpopulations of vascular-endothelial cells and cortical-neuroendocrine cells. Utilizing spatial transcriptome and pseudotime trajectory analysis, we support evidence of the centripetal dynamics of adrenocortical cell maintenance and the essential role played by Wnt/β-catenin, sonic hedgehog, and fibroblast growth factor pathways in the adult adrenocortical homeostasis. Furthermore, we compared single-nuclei transcriptional profiles obtained from six healthy adrenal glands and twelve adrenocortical adenomas. This analysis unveiled a notable heterogeneity in cell populations within the adenoma samples. In addition, we identified six distinct adenoma-specific clusters, each with varying distributions based on steroid profiles and tumour mutational status.
Overall, our results provide novel insights into adrenal homeostasis and molecular mechanisms potentially underlying early adrenocortical tumorigenesis and/or autonomous steroid secretion. Our cell atlas represents a powerful resource to investigate other adrenal-related pathologies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


