Interpretable machine learning models based on shear-wave elastography radiomics for predicting cardiovascular disease in diabetic kidney disease patients
Abstract
Background
The risk of cardiovascular complications is significantly elevated in patients with diabetic kidney disease (DKD). Recognizing the link between the progression of DKD and an increased risk of cardiovascular disease (CVD), it is crucial to focus on the early prediction and management of CVD risk factors among these patients to potentially enhance their health outcomes.
Objective
This study sought to bridge the existing gap by developing and validating machine learning (ML) models that utilize clinical data and shear wave elastography (SWE) radiomics features to identify patients at risk of CVD, ultimately aiming to improve the management of DKD.
Materials and Methods
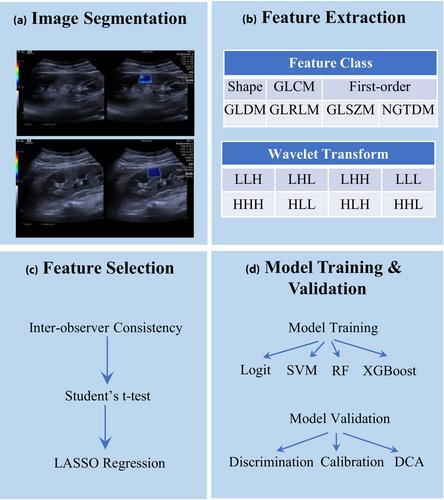
This study conducted a retrospective analysis of 586 patients with DKD, dividing them into training and external validation cohorts. We categorized patients based on the presence or absence of CVD. Utilizing SWE imaging, we extracted and standardized radiomics features to develop multiple ML models. These models underwent internal validation using radiomics features alone, clinical data, or a combination thereof. The optimal model was then identified, and its feature importance was assessed through the Shapley Additive Explanations (SHAP) method, before proceeding to external validation.
Results
Among the 586 patients analyzed, 30.7% (180/586) were identified as at risk for CVD. The study pinpointed six significant radiomics features related to CVD, alongside six critical pieces of clinical data. The Support Vector Machine (SVM) model outperformed others in both internal and external validations. Further, SHAP analysis highlighted five principal determinants of CVD risk, comprising three clinical indicators and two SWE radiomics features.
Conclusions
This study highlights the effectiveness of an SVM model that combines clinical and radiomics features in predicting CVD risk among DKD patients. It enables early prediction of CVD in this patient group, thereby supporting the implementation of timely and suitable interventions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


