Self-stacked small molecules for ultrasensitive, substrate-free Raman imaging in vivo
IF 41.7
1区 生物学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
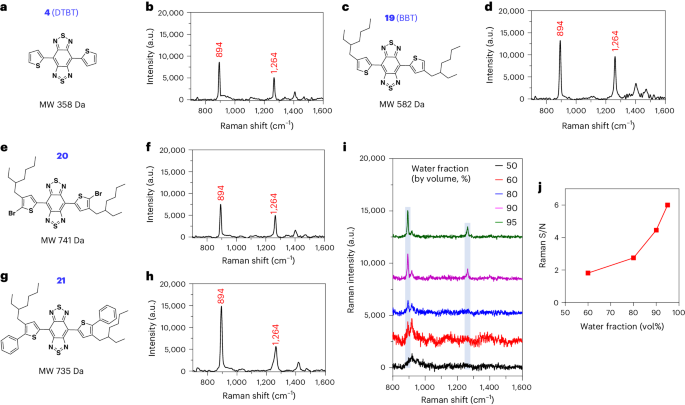
Raman spectroscopy using surface-enhanced Raman scattering (SERS) nanoprobes represents an ultrasensitive and high-precision technique for in vivo imaging. Clinical translation of SERS nanoprobes has been hampered by biosafety concerns about the metal substrates used to enhance Raman signals. We report a set of small molecules with bis-thienyl-substituted benzobisthiadiazole structures that enhance Raman signal through self-stacking rather than external substrates. In our technique, called stacking-induced charge transfer-enhanced Raman scattering (SICTERS), the self-stacked small molecules form an ordered spatial arrangement that enables three-dimensional charge transfer between neighboring molecules. The Raman scattering cross-section of SICTERS nanoprobes is 1350 times higher than that of conventional SERS gold nanoprobes of similar particle size. SICTERS outperforms SERS in terms of in vivo imaging sensitivity, resolution and depth. SICTERS is capable of noninvasive Raman imaging of blood and lymphatic vasculatures, which has not been achieved by SERS. SICTERS represents an alternative technique to enhance Raman scattering for guiding the design of ultrasensitive substrate-free Raman imaging probes. The sensitivity of in vivo Raman imaging is improved with self-stacked small molecules.

用于体内超灵敏、无基底拉曼成像的自堆积小分子
使用表面增强拉曼散射(SERS)纳米探针进行拉曼光谱分析是一种超灵敏、高精度的体内成像技术。SERS 纳米探针的临床应用一直受到用于增强拉曼信号的金属基底的生物安全性问题的阻碍。我们报告了一组具有双噻吩基取代的苯并双噻二唑结构的小分子,它们通过自堆叠而不是外部基底来增强拉曼信号。我们的技术被称为 "堆叠诱导电荷转移增强拉曼散射(SICTERS)",自堆叠小分子形成了有序的空间排列,从而实现了相邻分子之间的三维电荷转移。SICTERS 纳米探针的拉曼散射截面是粒径相似的传统 SERS 金纳米探针的 1350 倍。SICTERS 在体内成像灵敏度、分辨率和深度方面都优于 SERS。SICTERS 能够对血液和淋巴管进行无创拉曼成像,这是 SERS 所无法实现的。SICTERS 是增强拉曼散射的另一种技术,可用于指导超灵敏无基底拉曼成像探针的设计。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature biotechnology
工程技术-生物工程与应用微生物
CiteScore
63.00
自引率
1.70%
发文量
382
审稿时长
3 months
期刊介绍:
Nature Biotechnology is a monthly journal that focuses on the science and business of biotechnology. It covers a wide range of topics including technology/methodology advancements in the biological, biomedical, agricultural, and environmental sciences. The journal also explores the commercial, political, ethical, legal, and societal aspects of this research.
The journal serves researchers by providing peer-reviewed research papers in the field of biotechnology. It also serves the business community by delivering news about research developments. This approach ensures that both the scientific and business communities are well-informed and able to stay up-to-date on the latest advancements and opportunities in the field.
Some key areas of interest in which the journal actively seeks research papers include molecular engineering of nucleic acids and proteins, molecular therapy, large-scale biology, computational biology, regenerative medicine, imaging technology, analytical biotechnology, applied immunology, food and agricultural biotechnology, and environmental biotechnology.
In summary, Nature Biotechnology is a comprehensive journal that covers both the scientific and business aspects of biotechnology. It strives to provide researchers with valuable research papers and news while also delivering important scientific advancements to the business community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


