CCKBR+ cancer cells contribute to the intratumor heterogeneity of gastric cancer and confer sensitivity to FOXO inhibition
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
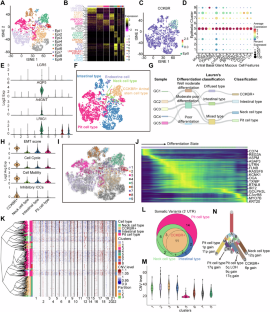
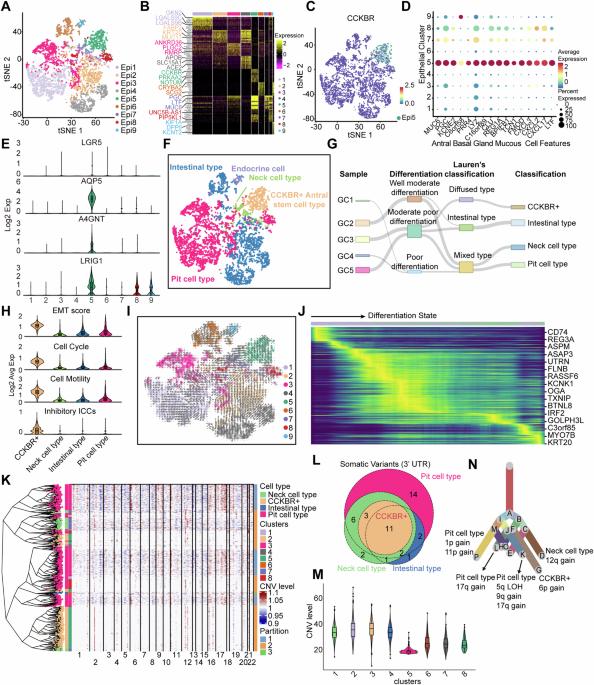
The existence of heterogeneity has plunged cancer treatment into a challenging dilemma. We profiled malignant epithelial cells from 5 gastric adenocarcinoma patients through single-cell sequencing (scRNA-seq) analysis, demonstrating the heterogeneity of gastric adenocarcinoma (GA), and identified the CCKBR+ stem cell-like cancer cells associated poorly differentiated and worse prognosis. We further conducted targeted analysis using single-cell transcriptome libraries, including 40 samples, to confirm these screening results. In addition, we revealed that FOXOs are involved in the progression and development of CCKBR+ gastric adenocarcinoma. Inhibited the expression of FOXOs and disrupting cancer cell stemness reduce the CCKBR+ GA organoid formation and impede tumor progression. Mechanically, CUT&Tag sequencing and Lectin pulldown revealed that FOXOs can activate ST3GAL3/4/5 as well as ST6GALNAC6, promoting elevated sialyation levels in CCKBR+ tumor cells. This FOXO-sialyltransferase axis contributes to the maintenance of homeostasis and the growth of CCKBR+ tumor cells. This insight provides novel perspectives for developing targeted therapeutic strategies aimed at the treating CCKBR associated gastric cancer.
CCKBR+癌细胞导致胃癌的瘤内异质性,并赋予其对 FOXO 抑制剂的敏感性。
异质性的存在使癌症治疗陷入两难境地。我们通过单细胞测序(scRNA-seq)分析了5例胃腺癌患者的恶性上皮细胞,证明了胃腺癌(GA)的异质性,并确定了CCKBR+干细胞样癌细胞与分化差和预后较差有关。我们进一步利用单细胞转录组文库(包括 40 个样本)进行了靶向分析,以证实这些筛选结果。此外,我们还发现 FOXOs 参与了 CCKBR+ 胃腺癌的进展和发展。抑制 FOXOs 的表达和破坏癌细胞干性可减少 CCKBR+ GA 器官样癌的形成并阻碍肿瘤的进展。从机理上讲,CUT&Tag测序和Lectin pulldown发现,FOXO能激活ST3GAL3/4/5和ST6GALNAC6,促进CCKBR+肿瘤细胞中的苷元化水平升高。这一 FOXO-氨酰基转移酶轴有助于维持 CCKBR+ 肿瘤细胞的平衡和生长。这一观点为开发旨在治疗 CCKBR 相关胃癌的靶向治疗策略提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


