Efficacy and safety of bosutinib in previously treated patients with chronic myeloid leukemia: final results from the BYOND trial
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
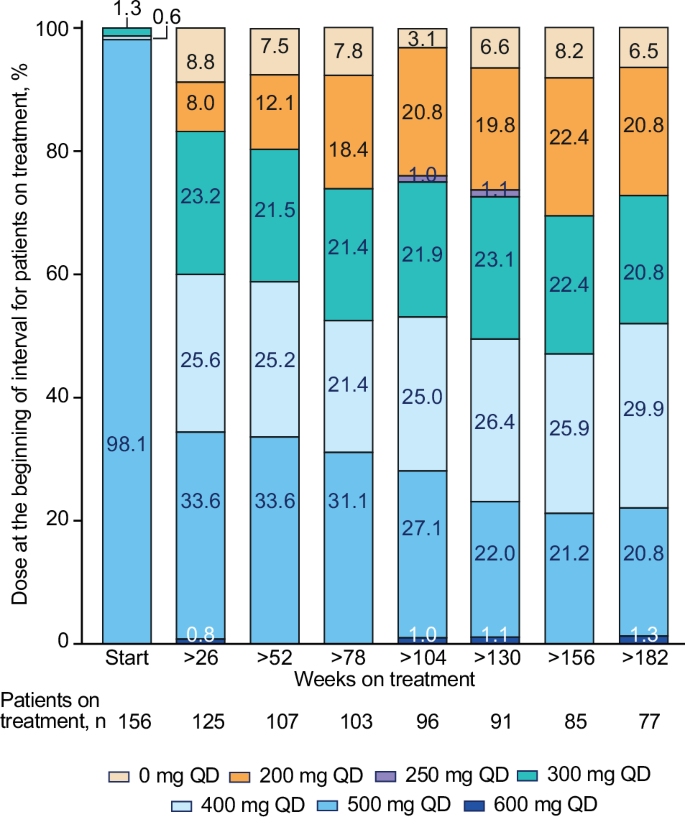
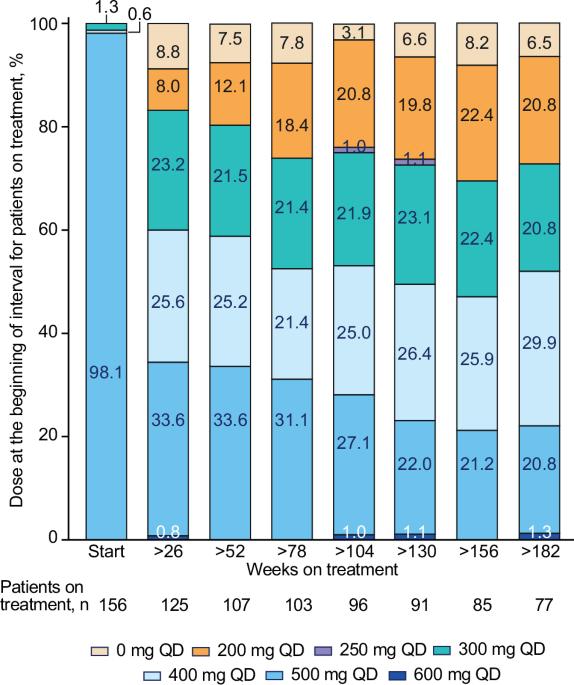
This final analysis from the phase 4 BYOND trial reports outcomes with bosutinib in patients with previously treated chronic myeloid leukemia (CML); 163 patients with CML resistant/intolerant to previous tyrosine kinase inhibitors received bosutinib (starting dose: 500 mg QD). At study completion (median follow-up, 47.8 months), 48.1% (n = 75/156) of patients with Philadelphia chromosome–positive chronic phase CML were still receiving treatment. Among evaluable patients, 71.8% (95% CI, 63.9–78.9) and 59.7% (95% CI, 51.4–67.7) attained or maintained major molecular response (MMR) and molecular response (MR)4, respectively, at any time on treatment. The majority of patients achieved a deeper molecular response relative to baseline while on bosutinib. Kaplan-Meier probabilities (95% CI) of maintaining MMR and MR4 at 36 months were 87.2% (78.0–92.7) and 80.7% (69.4–88.1), respectively. At 48 months, the Kaplan-Meier overall survival rate was 88.3% (95% CI, 81.8–92.6); there were 17 deaths, including 2 that were considered CML related. Long-term adverse events (AEs) were consistent with the known safety profile of bosutinib, and no new safety issues were identified. The management of AEs through dose reduction maintained efficacy while improving tolerability. These results support the use of bosutinib in patients with previously treated CML. ClinicalTrials.gov, NCT02228382
博舒替尼对既往接受过治疗的慢性髓性白血病患者的疗效和安全性:BYOND 试验的最终结果
这项4期BYOND试验的最终分析报告了博舒替尼在既往接受过治疗的慢性髓性白血病(CML)患者中的疗效;163名对既往酪氨酸激酶抑制剂耐药/不耐受的CML患者接受了博舒替尼(起始剂量:500 mg QD)治疗。研究结束时(中位随访47.8个月),48.1%(n=75/156)的费城染色体阳性慢性期CML患者仍在接受治疗。在可评估的患者中,71.8%(95% CI,63.9-78.9)和59.7%(95% CI,51.4-67.7)的患者在接受治疗的任何时间分别达到或维持了主要分子反应(MMR)和分子反应(MR)4。与基线相比,大多数患者在服用博舒替尼期间都获得了更深层次的分子反应。在36个月时,保持MMR和MR4的卡普兰-梅耶概率(95% CI)分别为87.2%(78.0-92.7)和80.7%(69.4-88.1)。48个月时,Kaplan-Meier总生存率为88.3%(95% CI,81.8-92.6);有17人死亡,其中2人被认为与CML有关。长期不良事件(AEs)与博苏替尼的已知安全性一致,未发现新的安全性问题。通过减少剂量来控制 AEs,既保持了疗效,又改善了耐受性。这些结果支持博舒替尼用于既往接受过治疗的CML患者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


