MiR-183-5p inhibits lung squamous cell carcinoma survival through disrupting hypoxia adaptation mediated by HIF-1α/NDUFA4L2 axis
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Hypoxia is a common feature of lung squamous cell carcinoma (LUSC), and hypoxia-inducible factor-1 (HIF-1) overexpression is associated with poor clinical outcome in LUSC. NADH dehydrogenase 1 alpha subcomplex subunit 4-like 2 (NDUFA4L2) is a recently identified target of HIF-1, but its roles in LUSC remain unclear. Herein, the expression and regulatory mechanisms of NDUFA4L2 were investigated in LUSC, and the influences on LUSC cell oxidative metabolism and survival of NDUFA4L2 were determined. The potential microRNA targeting to NDUFA4L2 was identified and its roles on LUSC cell were detected. We found that NDUFA4L2 were overexpressed in LUSC tissues, and that NDUFA4L2 expression correlated with shorter overall survival. NDUFA4L2 was regulated by HIF-1α under hypoxia, and NDUFA4L2 decreased mitochondrial reactive oxygen species (mitoROS) production through inhibiting mitochondrial complex I activity in LUSC cells. NDUFA4L2 silencing effectively suppressed LUSC cell growth and enhanced apoptosis by inducing mitoROS accumulation. Additionally, NDUFA4L2 was a target for miR-183-5p, and LUSC patients with high miR-183-5p levels had better prognoses. MiR-183-5p significantly induced mitoROS production and suppressed LUSC survival through negatively regulating NDUFA4L2 in vitro and in vivo. Our results suggested that regulation of NDUFA4L2 by HIF-1α is an important mechanism promoting LUSC progression under hypoxia. NDUFA4L2 inhibition using enforced miR-183-5p expression might be an effective strategy for LUSC treatment.
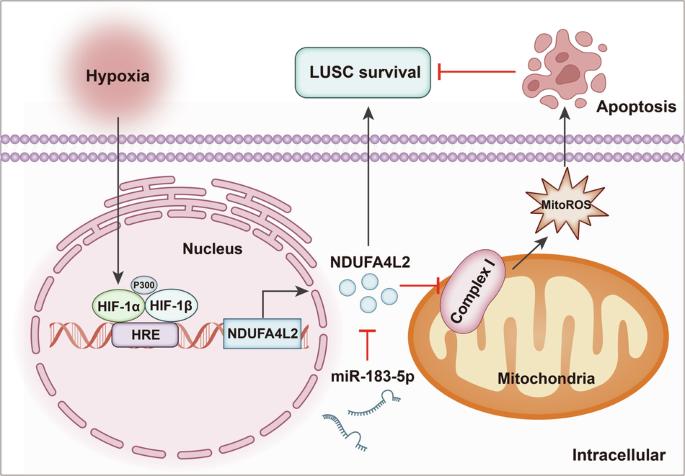
MiR-183-5p通过破坏由HIF-1α/NDUFA4L2轴介导的低氧适应,抑制肺鳞癌的存活。
缺氧是肺鳞癌(LUSC)的常见特征,而缺氧诱导因子-1(HIF-1)的过度表达与肺鳞癌的不良临床预后有关。NADH dehydrogenase 1 alpha subcomplex subunit 4-like 2 (NDUFA4L2)是最近发现的HIF-1靶点,但其在LUSC中的作用仍不清楚。本文研究了NDUFA4L2在LUSC中的表达和调控机制,并确定了NDUFA4L2对LUSC细胞氧化代谢和存活的影响。确定了NDUFA4L2的潜在靶向microRNA,并检测了其对LUSC细胞的作用。我们发现,NDUFA4L2在LUSC组织中过表达,且NDUFA4L2的表达与较短的总生存期相关。在缺氧条件下,NDUFA4L2受HIF-1α调控,NDUFA4L2通过抑制LUSC细胞线粒体复合物I的活性减少线粒体活性氧(mitoROS)的产生。沉默 NDUFA4L2 能有效抑制 LUSC 细胞的生长,并通过诱导线粒体活性氧的积累促进细胞凋亡。此外,NDUFA4L2是miR-183-5p的靶点,miR-183-5p水平高的LUSC患者预后更好。miR-183-5p通过负向调节NDUFA4L2,在体外和体内都能明显诱导线粒体ROS的产生,抑制LUSC的存活。我们的研究结果表明,HIF-1α对NDUFA4L2的调控是缺氧条件下促进LUSC进展的重要机制。利用miR-183-5p的表达抑制NDUFA4L2可能是治疗LUSC的有效策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


