Jumonji histone demethylases are therapeutic targets in small cell lung cancer
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
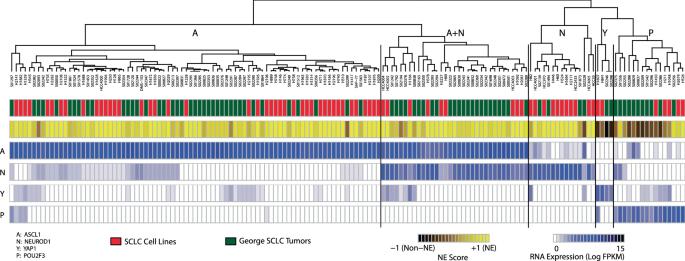
Small cell lung cancer (SCLC) is a recalcitrant cancer of neuroendocrine (NE) origin. Changes in therapeutic approaches against SCLC have been lacking over the decades. Here, we use preclinical models to identify a new therapeutic vulnerability in SCLC consisting of the targetable Jumonji lysine demethylase (KDM) family. We show that Jumonji demethylase inhibitors block malignant growth and that etoposide-resistant SCLC cell lines are particularly sensitive to Jumonji inhibition. Mechanistically, small molecule-mediated inhibition of Jumonji KDMs activates endoplasmic reticulum (ER) stress genes, upregulates ER stress signaling, and triggers apoptotic cell death. Furthermore, Jumonji inhibitors decrease protein levels of SCLC NE markers INSM1 and Secretogranin-3 and of driver transcription factors ASCL1 and NEUROD1. Genetic knockdown of KDM4A, a Jumonji demethylase highly expressed in SCLC and a known regulator of ER stress genes, induces ER stress response genes, decreases INSM1, Secretogranin-3, and NEUROD1 and inhibits proliferation of SCLC in vitro and in vivo. Lastly, we demonstrate that two different small molecule Jumonji KDM inhibitors (pan-inhibitor JIB-04 and KDM4 inhibitor SD70) block the growth of SCLC tumor xenografts in vivo. Our study highlights the translational potential of Jumonji KDM inhibitors against SCLC, a clinically feasible approach in light of recently opened clinical trials evaluating this drug class, and establishes KDM4A as a relevant target across SCLC subtypes.
Jumonji组蛋白去甲基化酶是小细胞肺癌的治疗靶点。
小细胞肺癌(SCLC)是一种神经内分泌(NE)起源的顽固性癌症。几十年来,针对小细胞肺癌的治疗方法一直缺乏变化。在这里,我们利用临床前模型确定了由可靶向的 Jumonji 赖氨酸去甲基化酶(KDM)家族组成的 SCLC 新的治疗漏洞。我们的研究表明,Jumonji 去甲基化酶抑制剂能阻止恶性生长,而对依托泊苷耐药的 SCLC 细胞系对 Jumonji 抑制剂特别敏感。从机理上讲,小分子介导的Jumonji KDMs抑制剂会激活内质网(ER)应激基因,上调ER应激信号,并引发细胞凋亡。此外,Jumonji抑制剂还能降低SCLC NE标志物INSM1和Secretogranin-3以及驱动转录因子ASCL1和NEUROD1的蛋白水平。KDM4A是一种在SCLC中高度表达的Jumonji去甲基化酶,也是已知的ER应激基因调控因子,基因敲除KDM4A会诱导ER应激反应基因,降低INSM1、Secretogranin-3和NEUROD1的水平,并抑制SCLC在体外和体内的增殖。最后,我们证明了两种不同的小分子 Jumonji KDM 抑制剂(泛抑制剂 JIB-04 和 KDM4 抑制剂 SD70)可阻断 SCLC 肿瘤异种移植物在体内的生长。我们的研究强调了Jumonji KDM抑制剂对SCLC的转化潜力,鉴于最近开始的临床试验对这类药物进行了评估,这是一种临床上可行的方法,并确定了KDM4A是SCLC亚型的一个相关靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


