Altered structural network in temporal lobe epilepsy with focal to bilateral tonic–clonic seizures
Abstract
Objectives
This study aims to investigate whether alterations in white matter topological networks are associated with focal to bilateral tonic–clonic seizures (FBTCS) in temporal lobe epilepsy (TLE). Additionally, we investigated the variables contributing to memory impairment in TLE.
Methods
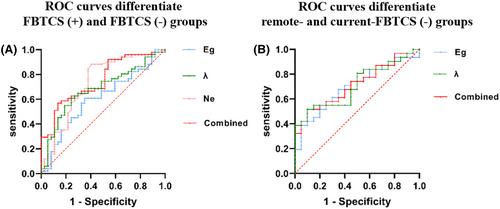
This cross-sectional study included 88 unilateral people with TLE (45 left/43 right), and 42 healthy controls. Graph theory analysis was employed to compare the FBTCS (+) group (n = 51) with the FBTCS (−) group (n = 37). The FBTCS (+) group was subcategorized into current-FBTCS (n = 31) and remote-FBTCS (n = 20), based on the history of FBTCS within 1 year or longer than 1 year before scanning, respectively. We evaluated the discriminatory power of topological network properties by receiver operating characteristic (ROC) analysis. Generalized linear models (GLMs) were employed to investigate variables associated with memory impairment in TLE.
Results
Global efficiency (Eg) was significantly reduced in the FBTCS (+) group, especially in the current-FBTCS subgroup. Greater disruption of regional properties in the ipsilateral occipital and temporal association cortices was observed in the FBTCS (+) group. ROC analysis revealed that Eg, normalized characteristic shortest path length, and nodal efficiency of the ipsilateral middle temporal gyrus could distinguish between FBTCS (+) and FBTCS (−) groups. Additionally, GLMs linked the occurrence of current FBTCS with poorer verbal memory outcomes in TLE.
Interpretation
Our study suggests that abnormal networks could be the structural basis of seizure propagation in FBTCS. Strategies aimed at reducing the occurrence of FBTCS could potentially improve the memory outcomes in people with TLE.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


