Dynamic Relapse Prediction by Peripheral Blood WT1mRNA after Allogeneic Hematopoietic Cell Transplantation for Myeloid Neoplasms
IF 3.6
3区 医学
Q2 HEMATOLOGY
引用次数: 0
Abstract
Although various relapse prediction models based on pretransplant information have been reported, they cannot update the predictive probability considering post-transplant patient status. Therefore, these models are not appropriate for deciding on treatment adjustment and preemptive intervention during post-transplant follow-up. A dynamic prediction model can update the predictive probability by considering the information obtained during follow-up. This study aimed to develop and assess a dynamic relapse prediction model after allogeneic hematopoietic cell transplantation (allo-HCT) for acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) using peripheral blood Wilms’ tumor 1 messenger RNA (WT1mRNA). We retrospectively analyzed patients with AML or MDS who underwent allo-HCT at our institution. To develop dynamic models, we employed the landmarking supermodel approach, using age, refined disease risk index, conditioning intensity, and number of transplantations as pretransplant covariates and both pre- and post-transplant peripheral blood WT1mRNA levels as time-dependent covariates. Finally, we compared the predictive performances of the conventional and dynamic models by area under the time-dependent receiver operating characteristic curves. A total of 238 allo-HCT cases were included in this study. The dynamic model that considered all pretransplant WT1mRNA levels and their kinetics showed superior predictive performance compared to models that considered only pretransplant covariates or factored in both pretransplant covariates and post-transplant WT1mRNA levels without their kinetics; their time-dependent areas under the curve were 0.89, 0.73, and 0.87, respectively. The predictive probability of relapse increased gradually from approximately 90 days before relapse. Furthermore, we developed a web application to make our model user-friendly. This model facilitates real-time, highly accurate, and personalized relapse prediction at any time point after allo-HCT. This will aid decision-making during post-transplant follow-up by offering objective relapse forecasts for physicians.
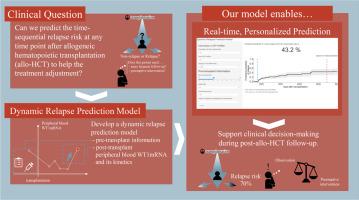
通过外周血 WT1mRNA 预测骨髓肿瘤异基因造血细胞移植后的动态复发。
背景:尽管有报道称各种基于移植前信息的复发预测模型,但这些模型无法根据移植后患者的状况更新预测概率。因此,这些模型不适合在移植后随访期间决定治疗调整和预先干预。动态预测模型可通过考虑随访期间获得的信息更新预测概率:本研究旨在利用外周血Wilms' tumor 1信使RNA(WT1mRNA),开发并评估急性髓性白血病(AML)和骨髓增生异常综合征(MDS)异基因造血细胞移植(allo-HCT)后的动态复发预测模型:我们回顾性地分析了在本院接受allo-HCT的AML或MDS患者。为了建立动态模型,我们采用了地标超模型方法,将年龄、精细疾病风险指数、调理强度和移植次数作为移植前协变量,将移植前和移植后外周血 WT1mRNA 水平作为时间依赖性协变量。最后,我们通过时间依赖性接收者操作特征曲线下面积比较了传统模型和动态模型的预测性能:本研究共纳入 238 例异体肝移植病例。与只考虑移植前协变量或同时考虑移植前协变量和移植后 WT1mRNA 水平但不考虑其动力学的模型相比,考虑移植前所有 WT1mRNA 水平及其动力学的动态模型显示出更优越的预测性能;其时间依赖性曲线下面积分别为 0.89、0.73 和 0.87。复发的预测概率从复发前约 90 天开始逐渐增加。此外,我们还开发了一个网络应用程序,使我们的模型便于用户使用:该模型有助于在异体肝移植后的任何时间点进行实时、高度准确和个性化的复发预测。通过为医生提供客观的复发预测,这将有助于移植后随访期间的决策。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Transplantation and Cellular Therapy
Medicine-Hematology
CiteScore
7.00
自引率
15.60%
发文量
1061
审稿时长
51 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


