scRNA‐seq reveals the landscape of immune repertoire of PBMNCs in iMCD
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
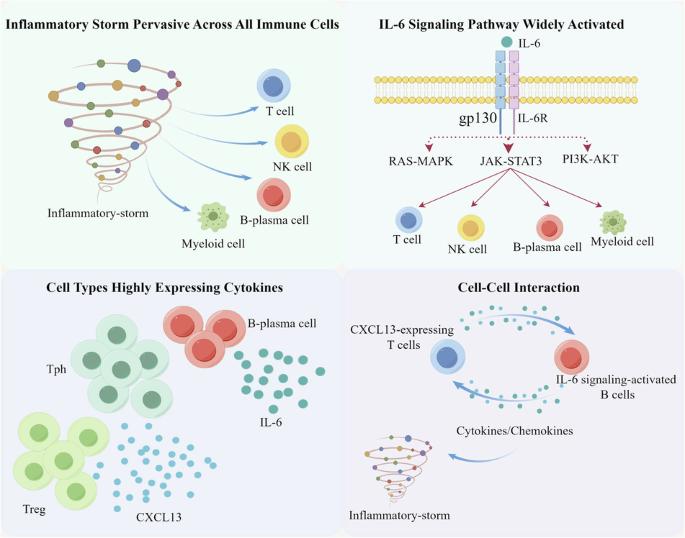
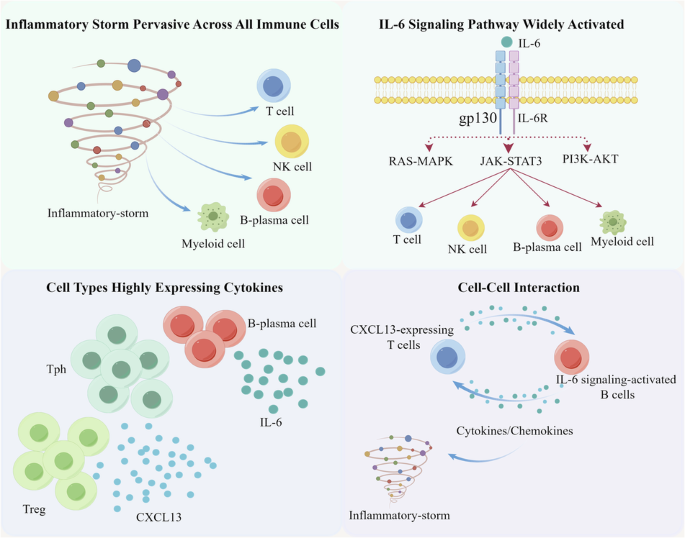
The etiology of idiopathic multicentric Castleman disease (iMCD) is poorly understood, and the identification of targetable disease mediators remains an unmet clinical need. Thus, we firstly employed single-cell RNA sequencing (scRNA-seq) to elucidate the landscape of the immune repertoire of peripheral blood mononuclear cells (PBMNCs) in iMCD and to identify additional driver cytokines/cells/pathways to address IL-6 blockade-refractory cases. We revealed that the inflammatory cytokine storm observed in iMCD was a significant phenomenon pervasive across all immune cells. B-plasma cell subsets was the main source of IL-6. The IL-6 signaling pathway was significantly activated across a spectrum of immune cells. Systemic upregulation of CXCL13 is mainly driven by peripheral helper T (Tph) and regulatory T (Treg) cells. Notably, a significant positive interaction was observed between CXCL13-expressing T cells and IL‐6 signaling-activated B cells. This study provides an immune perspective on PBMNCs in iMCD at the single-cell level, unveiling pathways or targets characterized by atypical inflammatory expression that could potentially serve as promising candidates for therapeutic intervention in iMCD.
scRNA-seq 揭示了 iMCD 中 PBMNCs 的免疫谱系。
特发性多中心卡斯特曼病(iMCD)的病因尚不清楚,识别可靶向的疾病介质仍是一项尚未满足的临床需求。因此,我们首先采用了单细胞RNA测序(scRNA-seq)技术来阐明iMCD中外周血单核细胞(PBMNCs)的免疫谱系,并找出更多的驱动细胞因子/细胞/通路,以解决IL-6阻断剂难治性病例的问题。我们发现,在 iMCD 中观察到的炎性细胞因子风暴是一种普遍存在于所有免疫细胞中的重要现象。B浆细胞亚群是IL-6的主要来源。IL-6信号通路在一系列免疫细胞中被显著激活。CXCL13 的系统性上调主要由外周辅助性 T 细胞(Tph)和调节性 T 细胞(Treg)驱动。值得注意的是,在表达 CXCL13 的 T 细胞和 IL-6 信号激活的 B 细胞之间观察到了明显的正向相互作用。这项研究在单细胞水平上为 iMCD 中的 PBMNCs 提供了一个免疫视角,揭示了以非典型炎症表达为特征的通路或靶点,这些通路或靶点有可能成为 iMCD 治疗干预的候选靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


