PrPC controls epithelial-to-mesenchymal transition in EGFR-mutated NSCLC: implications for TKI resistance and patient follow-up
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
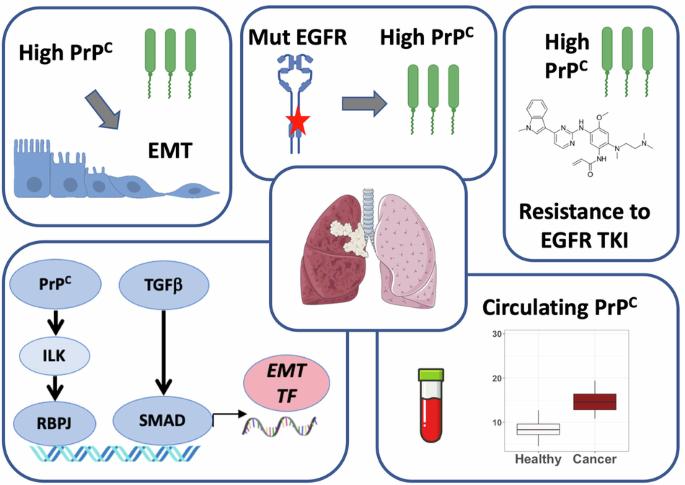
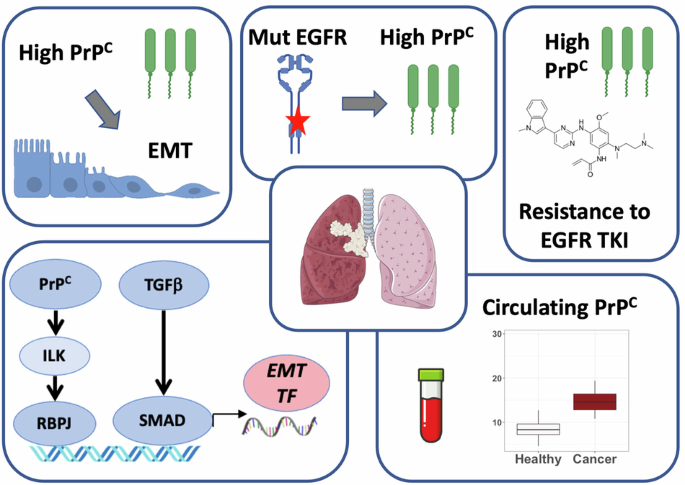
Patients with EGFR-mutated non-small cell lung cancer (NSCLC) benefit from treatment with tyrosine kinase inhibitors (TKI) targeting EGFR. Despite improvements in patient care, especially with the 3rd generation TKI osimertinib, disease relapse is observed in all patients. Among the various processes involved in TKI resistance, epithelial-to-mesenchymal transition (EMT) is far from being fully characterized. We hypothesized that the cellular prion protein PrPC could be involved in EMT and EGFR-TKI resistance in NSCLC. Using 5 independent lung adenocarcinoma datasets, including our own cohort, we document that the expression of the PRNP gene encoding PrPC is associated with EMT. By manipulating the levels of PrPC in different EGFR-mutated NSCLC cell lines, we firmly establish that the expression of PrPC is mandatory for cells to maintain or acquire a mesenchymal phenotype. Mechanistically, we show that PrPC operates through an ILK-RBPJ cascade, which also controls the expression of EGFR. Our data further demonstrate that PrPC levels are elevated in EGFR-mutated versus wild-type tumours or upon EGFR activation in vitro. In addition, we provide evidence that PRNP levels increase with TKI resistance and that reducing PRNP expression sensitizes cells to osimertinib. Finally, we found that plasma PrPC levels are increased in EGFR-mutated NSCLC patients from 2 independent cohorts and that their longitudinal evolution mirrors that of disease. Altogether, these findings define PrPC as a candidate driver of EMT-dependent resistance to EGFR-TKI in NSCLC. They further suggest that monitoring plasma PrPC levels may represent a valuable non-invasive strategy for patient follow-up and warrant considering PrPC-targeted therapies for EGFR-mutated NSCLC patients with TKI failure.
PrPC控制表皮生长因子受体突变的非小细胞肺癌的上皮-间质转化:对TKI耐药性和患者随访的影响。
表皮生长因子受体(EGFR)突变的非小细胞肺癌(NSCLC)患者可从针对EGFR的酪氨酸激酶抑制剂(TKI)治疗中获益。尽管患者护理有所改善,尤其是第三代 TKI 奥希替尼,但所有患者都会出现疾病复发。在TKI耐药所涉及的各种过程中,上皮细胞向间质转化(EMT)远未被完全定性。我们假设细胞朊病毒蛋白PrPC可能参与了NSCLC的EMT和表皮生长因子受体-TKI耐药。利用包括我们自己的队列在内的 5 个独立肺腺癌数据集,我们发现编码 PrPC 的 PRNP 基因的表达与 EMT 相关。通过调节不同的表皮生长因子受体突变 NSCLC 细胞系中 PrPC 的水平,我们证实 PrPC 的表达是细胞维持或获得间质表型的必要条件。从机理上讲,我们发现 PrPC 通过 ILK-RBPJ 级联发挥作用,而 ILK-RBPJ 级联也控制着表皮生长因子受体的表达。我们的数据进一步证明,与野生型肿瘤相比,表皮生长因子受体突变型肿瘤或表皮生长因子受体体外激活时,PrPC 水平会升高。此外,我们还提供了证据,证明 PRNP 水平会随着 TKI 抗性的增加而升高,而降低 PRNP 的表达可使细胞对奥希替尼敏感。最后,我们发现来自两个独立队列的表皮生长因子受体突变的 NSCLC 患者血浆中的 PrPC 水平会升高,而且其纵向演变与疾病相关。总之,这些发现将 PrPC 定义为 NSCLC 患者对 EGFR-TKI 的 EMT 依赖性耐药性的候选驱动因素。这些发现进一步表明,监测血浆PrPC水平可能是一种有价值的非侵入性患者随访策略,因此有必要考虑对TKI治疗失败的表皮生长因子受体突变NSCLC患者采用PrPC靶向疗法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


