A subset of dopamine receptor-expressing neurons in the nucleus accumbens controls feeding and energy homeostasis
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
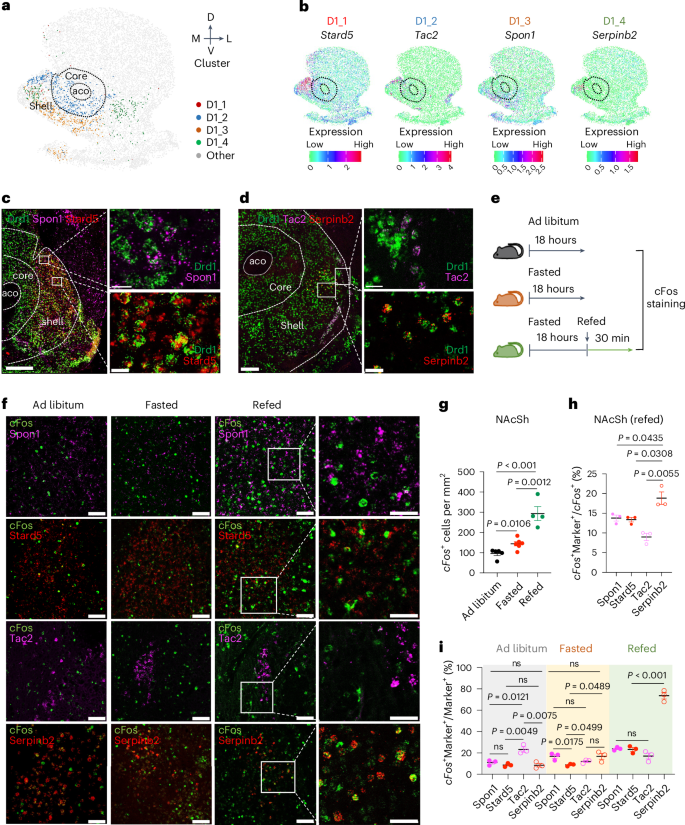
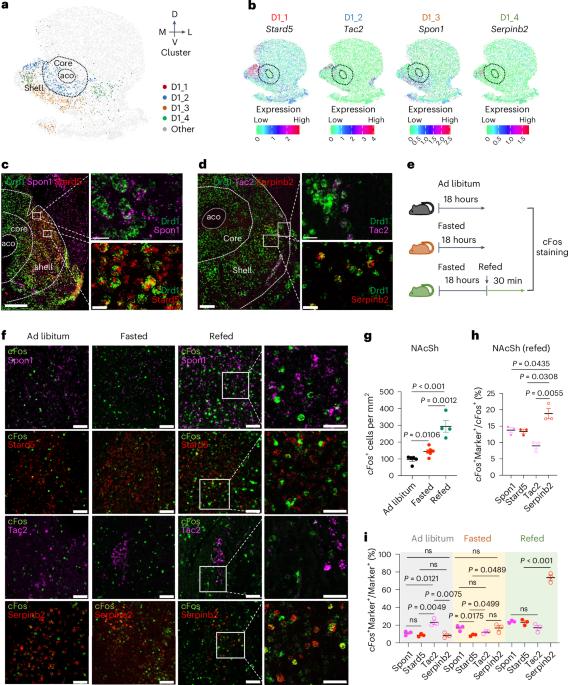
Orchestrating complex behaviors, such as approaching and consuming food, is critical for survival. In addition to hypothalamus neuronal circuits, the nucleus accumbens (NAc) also controls appetite and satiety. However, specific neuronal subtypes of the NAc that are involved and how the humoral and neuronal signals coordinate to regulate feeding remain incompletely understood. Here we decipher the spatial diversity of neuron subtypes of the NAc shell (NAcSh) and define a dopamine receptor D1-expressing and Serpinb2-expressing subtype controlling food consumption in male mice. Chemogenetics and optogenetics-mediated regulation of Serpinb2+ neurons bidirectionally regulate food seeking and consumption specifically. Circuitry stimulation reveals that the NAcShSerpinb2→LHLepR projection controls refeeding and can overcome leptin-mediated feeding suppression. Furthermore, NAcSh Serpinb2+ neuron ablation reduces food intake and upregulates energy expenditure, resulting in reduced bodyweight gain. Our study reveals a neural circuit consisting of a molecularly distinct neuronal subtype that bidirectionally regulates energy homeostasis, providing a potential therapeutic target for eating disorders. The work deciphers the spatial diversity of neuronal subtypes of the nucleus accumbens shell and identifies a subset of dopamine receptor-expressing neurons marked by Serpinb2 that control food seeking and consumption.
大脑凹陷核中表达多巴胺受体的神经元亚群控制进食和能量平衡
协调复杂的行为,如接近和摄取食物,对生存至关重要。除了下丘脑神经元回路外,纳氏核(NAc)也控制食欲和饱腹感。然而,人们对NAc神经元亚型的具体参与情况以及体液信号和神经元信号如何协调调节进食仍然知之甚少。在这里,我们破译了NAc外壳(NAcSh)神经元亚型的空间多样性,并确定了一个表达多巴胺受体D1和Serpinb2的亚型控制雄性小鼠的食量。化学遗传学和光遗传学介导的对Serpinb2+神经元的双向调控具体调节了食物的寻求和消耗。电路刺激显示,NAcShSerpinb2→LHLepR投射控制再摄食,并能克服瘦素介导的摄食抑制。此外,NAcSh Serpinb2+ 神经元消融可减少食物摄入量并增加能量消耗,从而减少体重增加。我们的研究揭示了一个由分子上不同的神经元亚型组成的神经回路,它能双向调节能量平衡,为进食障碍提供了一个潜在的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


