The VLA-4 integrin is constitutively active in circulating chronic lymphocytic leukemia cells via BCR autonomous signaling: a novel anchor-independent mechanism exploiting soluble blood-borne ligands
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
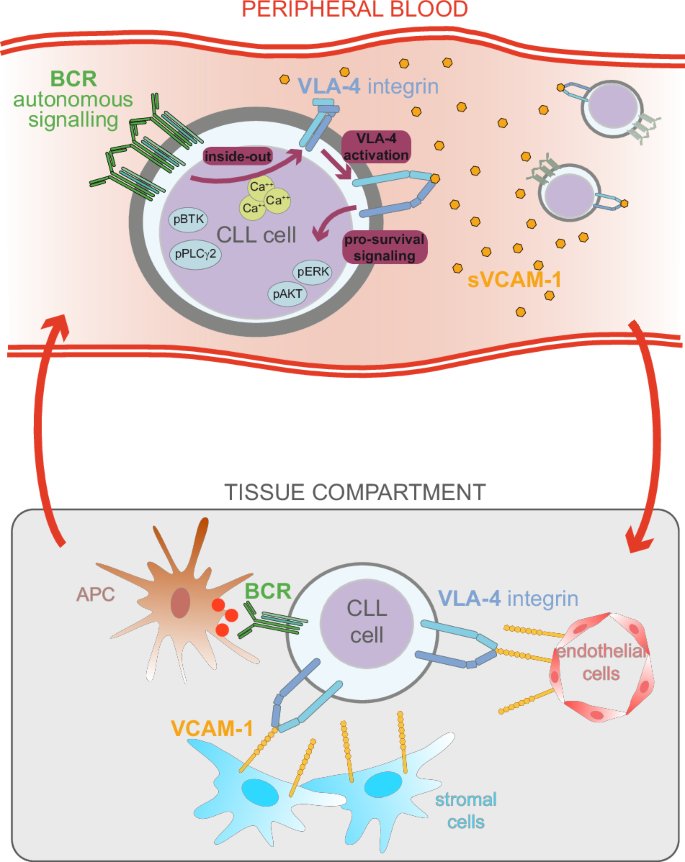
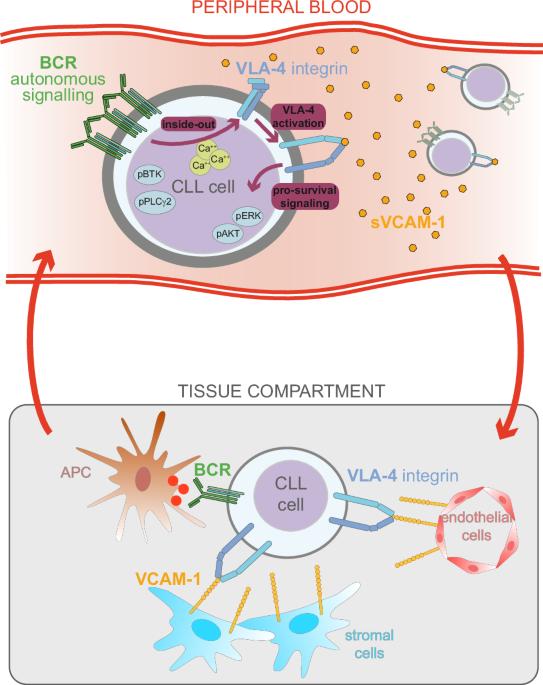
In chronic lymphocytic leukemia (CLL), survival of neoplastic cells depends on microenvironmental signals at lymphoid sites where the crosstalk between the integrin VLA-4 (CD49d/CD29), expressed in ~40% of CLL, and the B-cell receptor (BCR) occurs. Here, BCR engagement inside–out activates VLA-4, thus enhancing VLA-4-mediated adhesion of CLL cells, which in turn obtain pro-survival signals from the surrounding microenvironment. We report that the BCR is also able to effectively inside–out activate the VLA-4 integrin in circulating CD49d-expressing CLL cells through an autonomous antigen-independent BCR signaling. As a consequence, circulating CLL cells exhibiting activated VLA-4 express markers of BCR pathway activation (phospho-BTK and phospho-PLC-γ2) along with higher levels of phospho-ERK and phospho-AKT indicating parallel activation of downstream pathways. Moreover, circulating CLL cells expressing activated VLA-4 bind soluble blood-borne VCAM-1 leading to increased VLA-4-dependent actin polymerization/re-organization and ERK phosphorylation. Finally, evidence is provided that ibrutinib treatment, by affecting autonomous BCR signaling, impairs the constitutive VLA-4 activation eventually decreasing soluble VCAM-1 binding and reducing downstream ERK phosphorylation by circulating CLL cells. This study describes a novel anchor-independent mechanism occurring in circulating CLL cells involving the BCR and the VLA-4 integrin, which help to unravel the peculiar biological and clinical features of CD49d+ CLL.
通过 BCR 自主信号传导,VLA-4 整合素在循环慢性淋巴细胞白血病细胞中持续活跃:一种利用可溶性血源性配体的新型锚依赖机制
在慢性淋巴细胞白血病(CLL)中,肿瘤细胞的存活取决于淋巴部位的微环境信号,在这些部位,整合素 VLA-4(CD49d/CD29)与 B 细胞受体(BCR)之间会发生串联。在这里,BCR 从内向外参与激活了 VLA-4,从而增强了 VLA-4 介导的 CLL 细胞粘附,而 CLL 细胞又从周围微环境中获得了促生存信号。我们报告说,BCR 还能通过独立于抗原的 BCR 信号转导,有效地从内向外激活循环中表达 CD49d 的 CLL 细胞中的 VLA-4 整合素。因此,表现出活化的 VLA-4 的循环 CLL 细胞会表达 BCR 通路活化的标记物(磷酸化-BTK 和磷酸化-PLC-γ2)以及更高水平的磷酸化-ERK 和磷酸化-AKT,这表明下游通路被同时活化。此外,表达活化的 VLA-4 的循环 CLL 细胞会与可溶性血载 VCAM-1 结合,导致依赖 VLA-4 的肌动蛋白聚合/重组和 ERK 磷酸化增加。最后,研究提供的证据表明,伊布替尼治疗通过影响自主BCR信号传导,损害了组成型VLA-4活化,最终减少了可溶性VCAM-1的结合,降低了循环CLL细胞下游的ERK磷酸化。这项研究描述了循环CLL细胞中发生的一种新型锚依赖机制,涉及BCR和VLA-4整合素,有助于揭示CD49d+ CLL的特殊生物学和临床特征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


