Phosphorylation patterns in the AT1R C-terminal tail specify distinct downstream signaling pathways
IF 6.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Different ligands stabilize specific conformations of the angiotensin II type 1 receptor (AT1R) that direct distinct signaling cascades mediated by heterotrimeric G proteins or β-arrestin. These different active conformations are thought to engage distinct intracellular transducers because of differential phosphorylation patterns in the receptor C-terminal tail (the “barcode” hypothesis). Here, we identified the AT1R barcodes for the endogenous agonist AngII, which stimulates both G protein activation and β-arrestin recruitment, and for a synthetic biased agonist that only stimulates β-arrestin recruitment. The endogenous and β-arrestin–biased agonists induced two different ensembles of phosphorylation sites along the C-terminal tail. The phosphorylation of eight serine and threonine residues in the proximal and middle portions of the tail was required for full β-arrestin functionality, whereas phosphorylation of the serine and threonine residues in the distal portion of the tail had little influence on β-arrestin function. Similarly, molecular dynamics simulations showed that the proximal and middle clusters of phosphorylated residues were critical for stable β-arrestin–receptor interactions. These findings demonstrate that ligands that stabilize different receptor conformations induce different phosphorylation clusters in the C-terminal tail as barcodes to evoke distinct receptor-transducer engagement, receptor trafficking, and signaling.
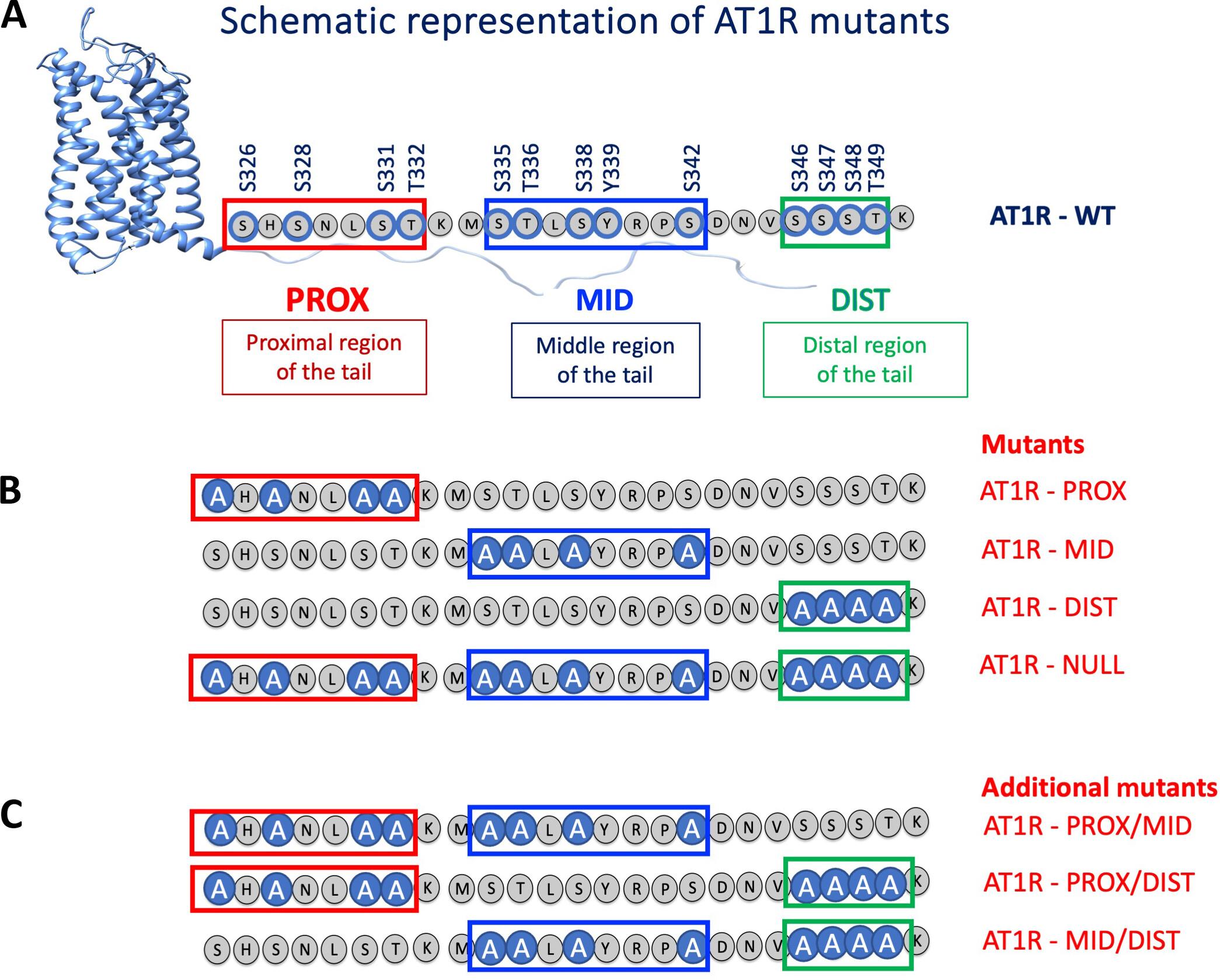
AT1R C 端尾部的磷酸化模式指定了不同的下游信号通路。
不同的配体会稳定血管紧张素 II 1 型受体(AT1R)的特定构象,从而引导由异源三聚体 G 蛋白或 β-阿司匹林介导的不同信号级联。由于受体 C 端尾部的磷酸化模式不同("条形码 "假说),这些不同的活性构象被认为与不同的细胞内转导因子有关。在这里,我们确定了同时刺激 G 蛋白活化和 β-restin 募集的内源性激动剂 AngII 和只刺激 β-restin 募集的合成偏激激动剂的 AT1R 条形码。内源性激动剂和β-阿restin偏激激动剂诱导了两种不同的 C 端尾部磷酸化位点组合。尾部近端和中间部分的 8 个丝氨酸和苏氨酸残基的磷酸化是完整的 β-阿restin 功能所必需的,而尾部远端丝氨酸和苏氨酸残基的磷酸化对 β-阿restin的功能影响很小。同样,分子动力学模拟显示,近端和中间的磷酸化残基簇对稳定的 β-restin-受体相互作用至关重要。这些研究结果表明,能稳定不同受体构象的配体会诱导 C 端尾部的不同磷酸化簇作为条形码,从而唤起不同的受体-转换器啮合、受体贩运和信号传导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Signaling
BIOCHEMISTRY & MOLECULAR BIOLOGY-CELL BIOLOGY
CiteScore
9.50
自引率
0.00%
发文量
148
审稿时长
3-8 weeks
期刊介绍:
"Science Signaling" is a reputable, peer-reviewed journal dedicated to the exploration of cell communication mechanisms, offering a comprehensive view of the intricate processes that govern cellular regulation. This journal, published weekly online by the American Association for the Advancement of Science (AAAS), is a go-to resource for the latest research in cell signaling and its various facets.
The journal's scope encompasses a broad range of topics, including the study of signaling networks, synthetic biology, systems biology, and the application of these findings in drug discovery. It also delves into the computational and modeling aspects of regulatory pathways, providing insights into how cells communicate and respond to their environment.
In addition to publishing full-length articles that report on groundbreaking research, "Science Signaling" also features reviews that synthesize current knowledge in the field, focus articles that highlight specific areas of interest, and editor-written highlights that draw attention to particularly significant studies. This mix of content ensures that the journal serves as a valuable resource for both researchers and professionals looking to stay abreast of the latest advancements in cell communication science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


