Adjunctive cenobamate in people with focal onset seizures: Insights from the Italian Expanded Access Program
Abstract
Objective
This study was undertaken to assess the effectiveness/tolerability of adjunctive cenobamate, variations in the load of concomitant antiseizure medications (ASMs) and predictors of clinical response in people with focal epilepsy.
Methods
This was a retrospective study at 21 centers participating in the Italian Expanded Access Program. Effectiveness outcomes included retention and responder rates (≥50% and 100% reduction in baseline seizure frequency). Tolerability/safety outcomes included the rate of treatment discontinuation due to adverse events (AEs) and their incidence. Total drug load was quantified as the number of concomitant ASMs and total defined daily dose (DDD). Concomitant ASMs were also classified according to their mechanism of action and pharmacokinetic interactions to perform explorative subgroup analyses.
Results
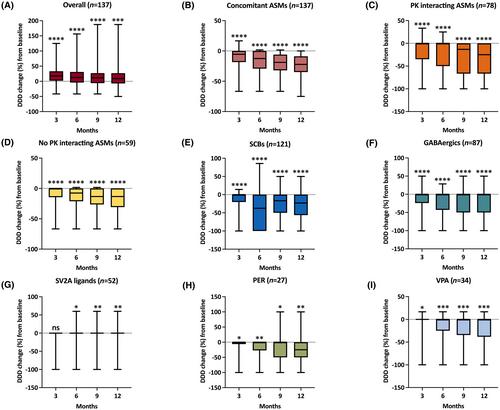
A total of 236 subjects with a median age of 38 (Q1–Q3 = 27–49) years were included. At 12 months, cenobamate retention rate was 78.8% and responders were 57.5%. The seizure freedom rates during the preceding 3 months were 9.8%, 12.2%, 16.3%, and 14.0% at 3, 6, 9, and 12 months. A higher percentage of responders was observed among subjects treated with clobazam, although the difference was not statistically significant. A total of 223 AEs were recorded in 133 of 236 participants, leading to cenobamate discontinuation in 8.5% cases. At 12 months, a reduction of one or two concomitant ASMs occurred in 42.6% and 4.3% of the subjects. The median total DDD of all concomitant ASMs decreased from 3.34 (Q1–Q3 = 2.50–4.47) at baseline to 2.50 (Q1–Q3 = 1.67–3.50) at 12 months (p < .001, median percentage reduction = 22.2%). The highest rates of cotreatment withdrawal and reductions in the DDD were observed for sodium channel blockers and γ-aminobutyric acidergic modulators (above all for those linked to pharmacokinetic interactions), and perampanel.
Significance
Adjunctive cenobamate was associated with a reduction in seizure frequency and in the burden of concomitant ASMs in adults with difficult-to-treat focal epilepsy. The type of ASM associated did not influence effectiveness except for a favorable trend with clobazam.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


