The AAV2.7m8 capsid packages a higher degree of heterogeneous vector genomes than AAV2
IF 4.5
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Recombinant adeno-associated virus (rAAV) vectors are currently the only proven vehicles for treating ophthalmological diseases through gene therapy. A wide range of gene therapy programs that target ocular diseases are currently being pursued. Nearly 20 years of research have gone into enhancing the efficacy of targeting retinal tissues and improving transgene delivery to specific cell types. The engineered AAV capsid, AAV2.7m8 is currently among the best capsids for transducing the retina following intravitreal (IVT) injection. However, adverse effects, including intraocular inflammation, have been reported following retinal administration of AAV2.7m8 vectors in clinical trials. Furthermore, we have consistently observed that AAV2.7m8 exhibits low packaging titers irrespective of the vector construct design. In this report, we found that AAV2.7m8 packages vector genomes with a higher degree of heterogeneity than AAV2. We also found that genome-loaded AAV2.7m8 stimulated the infiltration of microglia in mouse retinas following IVT administration, while the response to genome-loaded AAV2 and empty AAV2.7m8 capsids produced much milder responses. This finding suggests that IVT administration of AAV2.7m8 vectors may stimulate retinal immune responses in part because of its penchant to package and deliver non-unit length genomes.
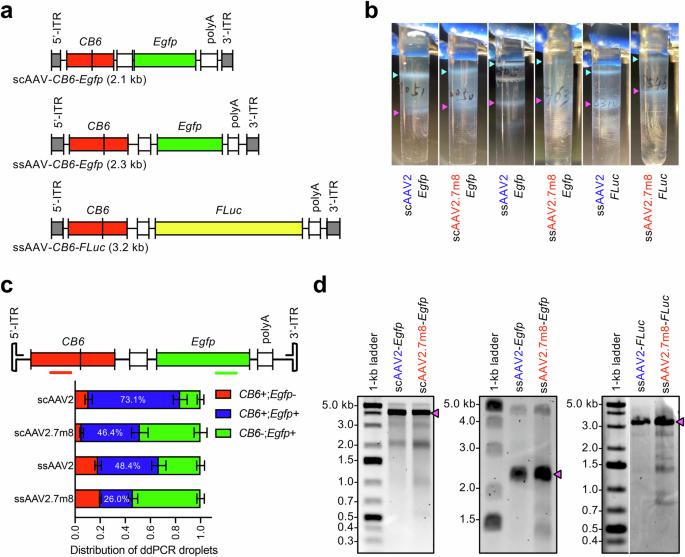
与 AAV2 相比,AAV2.7m8 的包囊包装异质载体基因组的程度更高。
重组腺相关病毒(rAAV)载体是目前通过基因疗法治疗眼科疾病的唯一行之有效的载体。目前,针对眼科疾病的基因治疗项目种类繁多。近 20 年来,人们一直在研究如何提高针对视网膜组织的疗效,以及如何改善转基因向特定细胞类型的传递。目前,AAV2.7m8 的工程化囊壳是玻璃体内注射(IVT)转导视网膜的最佳囊壳之一。然而,在临床试验中,AAV2.7m8 载体在视网膜内给药后出现了不良反应,包括眼内炎症。此外,我们一直观察到,无论载体构建设计如何,AAV2.7m8 都表现出较低的包装滴度。在本报告中,我们发现与 AAV2 相比,AAV2.7m8 包装载体基因组的异质性更高。我们还发现,IVT 给药后,装载基因组的 AAV2.7m8 会刺激小鼠视网膜中的小胶质细胞浸润,而装载基因组的 AAV2 和空 AAV2.7m8 胶囊产生的反应要轻微得多。这一发现表明,静脉注射AAV2.7m8载体可能会刺激视网膜免疫反应,部分原因是它喜欢包装和传递非单位长度的基因组。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


