The PRMT6/STAT1/ACSL1 axis promotes ferroptosis in diabetic nephropathy
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Hyperglycaemia-induced ferroptosis is a significant contributor to kidney dysfunction in diabetic nephropathy (DN) patients. In addition, targeting ferroptosis has clinical implications for the treatment of DN. However, effective therapeutic targets for ferroptosis have not been identified. In this study, we aimed to explore the precise role of protein arginine methyltransferase 6 (PRMT6) in regulating ferroptosis in DN. In the present study, we utilized a mouse DN model consisting of both wild-type and PRMT6-knockout (PRMT6−/−) mice. Transcriptomic and lipidomic analyses, along with various molecular biological methodologies, were used to determine the potential mechanism by which PRMT6 regulates ferroptosis in DN. Our results indicate that PRMT6 downregulation participates in kidney dysfunction and renal cell death via the modulation of ferroptosis in DN. Moreover, PRMT6 reduction induced lipid peroxidation by upregulating acyl-CoA synthetase long-chain family member 1 (ACSL1) expression, ultimately contributing to ferroptosis. Furthermore, we investigated the molecular mechanism by which PRMT6 interacts with signal transducer and activator of transcription 1 (STAT1) to jointly regulate ACSL1 transcription. Additionally, treatment with the STAT1-specific inhibitor fludarabine delayed DN progression. Furthermore, we observed that PRMT6 and STAT1 synergistically regulate ACSL1 transcription to mediate ferroptosis in hyperglycaemic cells. Our study demonstrated that PRMT6 and STAT1 comodulate ACSL1 transcription to induce the production of phospholipid-polyunsaturated fatty acids (PL-PUFAs), thus participating in ferroptosis in DN. These findings suggest that the PRMT6/STAT1/ACSL1 axis is a new therapeutic target for the prevention and treatment of DN.
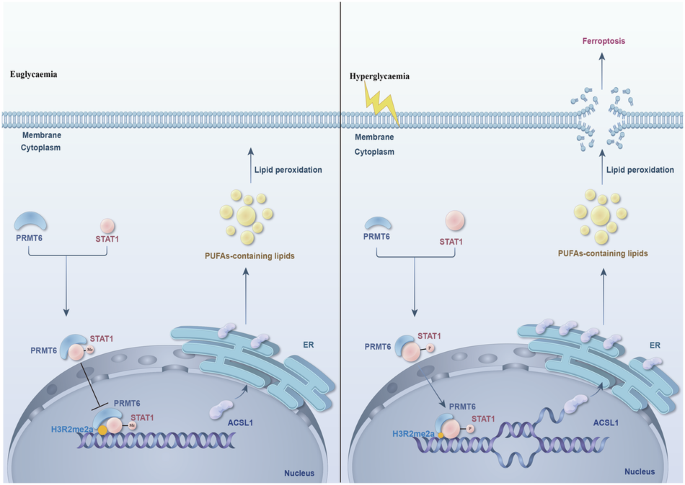
PRMT6/STAT1/ACSL1 轴促进糖尿病肾病中的铁蛋白沉积。
高血糖诱导的高铁血症是导致糖尿病肾病(DN)患者肾功能障碍的重要原因。此外,针对高铁血症的治疗对治疗糖尿病肾病也有临床意义。然而,目前尚未发现针对铁蛋白沉积的有效治疗靶点。在本研究中,我们旨在探索蛋白精氨酸甲基转移酶 6(PRMT6)在 DN 中调控铁凋亡的确切作用。在本研究中,我们使用了由野生型和 PRMT6 基因敲除(PRMT6-/-)小鼠组成的小鼠 DN 模型。通过转录组和脂质组分析以及各种分子生物学方法,我们确定了 PRMT6 在 DN 中调控铁突变的潜在机制。我们的研究结果表明,PRMT6 的下调通过调节 DN 中的铁蛋白沉积参与了肾功能障碍和肾细胞死亡。此外,PRMT6 下调通过上调酰基-CoA 合成酶长链家族成员 1(ACSL1)的表达诱导脂质过氧化,最终导致铁变态反应。此外,我们还研究了 PRMT6 与信号转导子和转录激活子 1(STAT1)相互作用共同调控 ACSL1 转录的分子机制。此外,用 STAT1 特异性抑制剂氟达拉滨治疗可延缓 DN 的进展。此外,我们还观察到 PRMT6 和 STAT1 能协同调控 ACSL1 的转录,从而介导高血糖细胞的铁变态反应。我们的研究表明,PRMT6和STAT1共同调控ACSL1转录,诱导磷脂-多不饱和脂肪酸(PL-PUFAs)的产生,从而参与了DN中的铁突变。这些研究结果表明,PRMT6/STAT1/ACSL1 轴是预防和治疗 DN 的一个新的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


