Method development: Assigning sex to African clawless otter spraints and assessing stability of faecal androgen and progestagen metabolites post-defaecation
Abstract
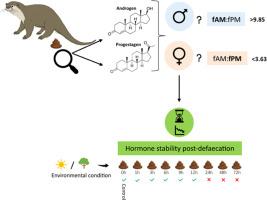
Monitoring reproductive physiology in wildlife can be a useful tool for assessing population dynamics for conservation and management purposes. Utilizing non-invasive approaches for this, such as quantifying reproductive hormone metabolites from faeces, can be challenging when defaecation events are not observed, or when cryptic species like African clawless otters (Aonyx capensis) are involved. Additionally, test systems for quantifying hormone metabolites in a species for the first time must first be reliably validated prior to use. Our results indicate that Epiandrosterone and Progesterone EIAs are most suitable for determining fAM and fPM concentrations in African clawless otter spraints. The fAM:fPM ratio and respective thresholds are more reliable in sex identification compared to the separate use of individual hormone classes. Sex-related hormone metabolite concentrations remained comparable for up to 12hrs post-defaecation in both sexes.
- •
We screened two androgen and two progestagen enzyme-immunoassays (EIAs) for suitability and reliable quantification of faecal androgen metabolites (fAM) and faecal progestagen metabolites (fPM) in African clawless otters.
- •
We assessed whether the ratio of fAM:fPM concentrations can be used to assign sex to faecal samples from unknown individuals.
- •
We tested the stability of fAM and fPM concentrations post-defaecation to determine the effects of environmental exposure and bacterial metabolism.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


