Palladium-Catalyzed anti-Michael-Type Hydroamination of N-(Quinolin-8-yl)acrylamide with 2-Pyridones
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Here we report a palladium-catalyzed anti-Michael-type hydroamination of N-(quinolin-8-yl)acrylamide with 2-pyridones. The use of a palladium catalyst enables the α-addition of 2-pyridones, resulting in the formation of a range of N-substituted 2-pyridone carboxamides with yields ranging from 10% to 80%. Derivatization of the products highlights the utility of this transformation. Preliminary mechanistic investigations suggest that the reaction proceeds through the direct addition of a nitrogen atom of 2-pyridones, ruling out other pathways such as O-to-N-alkyl migration.
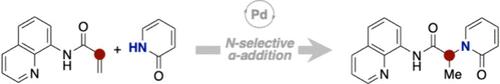
钯催化 N-(喹啉-8-基)丙烯酰胺与 2-吡啶酮的反迈克尔式加氢反应
在此,我们报告了钯催化 N-(喹啉-8-基)丙烯酰胺与 2-吡啶酮的反迈克尔型氢化反应。使用钯催化剂可以实现 2-吡啶酮的α-加成,从而形成一系列 N-取代的 2-吡啶酮羧酰胺,产率从 10% 到 80%不等。产物的衍生化凸显了这种转化的实用性。初步的机理研究表明,该反应是通过直接添加 2-吡啶酮的氮原子进行的,排除了 O-N-烷基迁移等其他途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


