Pharmacokinetic Bioequivalence between Generic and Originator Orally Inhaled Drug Products: Validity of Administration of Doses above the Approved Single Maximum Dose
IF 4.5
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
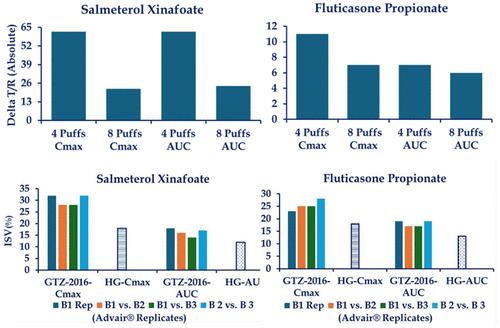
Pharmacokinetic bioequivalence of orally inhaled drug products is a critical component of the US FDA’s “weight of evidence” approach, and it can serve as the sole indicator of safety and effectiveness of follow-on inhalation products approved in Europe and some other geographic areas. The approved labels of the orally inhaled drug products recommend the maximum number of actuations that can be administered in a single dose on one occasion. This single maximum dose may consist of one or more inhalations depending upon the product. Bioequivalence studies for the inhalation drug product registrations in the US and EU have employed single and multiple actuation doses, in some cases over and above the approved single maximum labeled doses, thus, inconsistent with the approved labeling of the reference products. Pharmacokinetics of inhaled drug products after single and multiple doses may be different, with implications for bioequivalence determined at single and multiple doses. Scientific literature indicates that the relative bioavailability of the Test and Reference products may differ between administrations of doses in one and multiple inhalations. Multiple doses not only alter the pharmacokinetics but also may reduce the sensitivity of the bioassay to actual differences between the Test and Reference product performances. Ability of the pharmacokinetic bioassay to accurately determine the extent of difference between two products may also be substantially reduced at high doses. Therefore, in our opinion, pharmacokinetic bioequivalence to support regulatory approvals of inhalation products at doses above the recommended single maximum dose should be avoided. Furthermore, the bioequivalence of products (if any) established at doses exceeding the approved single maximum doses should be revisited to determine if the products maintain bioequivalence when evaluated at the clinically relevant single maximum doses.
仿制药与原研口服药物之间的药代动力学生物等效性:超过批准的单一最大剂量的给药有效性
口服吸入药物产品的药代动力学生物等效性是美国 FDA "证据权重 "方法的重要组成部分,也是欧洲和其他一些地区批准的后续吸入产品安全性和有效性的唯一指标。经批准的口服吸入药物产品标签推荐了单次给药的最大剂量。根据产品的不同,单次最大剂量可能包括一次或多次吸入。美国和欧盟的吸入式药物产品注册生物等效性研究采用了单次和多次给药剂量,在某些情况下超过了批准的单次最大标示剂量,因此与参考产品的批准标示不一致。单次和多次给药后,吸入药物产品的药代动力学可能会有所不同,这对确定单次和多次给药的生物等效性会产生影响。科学文献表明,试验品和参比品的相对生物利用度在单次吸入和多次吸入时可能有所不同。多剂量不仅会改变药代动力学,还可能降低生物测定对受试产品和参比产品实际性能差异的敏感性。高剂量时,药代动力学生物测定准确确定两种产品之间差异程度的能力也会大大降低。因此,我们认为,应避免以药代动力学生物等效性来支持监管部门对吸入产品的审批,其剂量应超过建议的单次最大剂量。此外,应重新审查以超过批准的单次最大剂量的剂量确定的产品(如有)的生物等效性,以确定产品在临床相关的单次最大剂量下进行评估时是否保持生物等效性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Pharmaceutics
医学-药学
CiteScore
8.00
自引率
6.10%
发文量
391
审稿时长
2 months
期刊介绍:
Molecular Pharmaceutics publishes the results of original research that contributes significantly to the molecular mechanistic understanding of drug delivery and drug delivery systems. The journal encourages contributions describing research at the interface of drug discovery and drug development.
Scientific areas within the scope of the journal include physical and pharmaceutical chemistry, biochemistry and biophysics, molecular and cellular biology, and polymer and materials science as they relate to drug and drug delivery system efficacy. Mechanistic Drug Delivery and Drug Targeting research on modulating activity and efficacy of a drug or drug product is within the scope of Molecular Pharmaceutics. Theoretical and experimental peer-reviewed research articles, communications, reviews, and perspectives are welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


