The critical role of the active [GaH]2+ site in n-heptane dehydrocyclization over Ga/H-ZSM-5 zeolite†
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The Ga/H-ZSM-5 zeolite has long been acknowledged as an effective catalyst for the aromatization of light alkanes. In the present work, a three-coordinated carbenium ion is identified as a crucial intermediate in the formation of the [GaH]2+ active site. In particular, the involvement of the carbenium ion as a bridge facilitates the kinetic unhindered creation and restoration of the [GaH]2+ active site. Using density functional theory calculations, comprehensive reaction pathways for n-heptane, encompassing its conversion to toluene via the C1–C6 ring closure, were explored for both H-ZSM-5 and Ga/H-ZSM-5 zeolites. Compared to the BAS, the [GaH]2+ active site significantly lowers the activation barrier for the C–H bond cleavage. Furthermore, Bader charge and crystal orbital Hamilton population analysis confirmed that the [GaH]2+ active site facilitates the activation of the C–H bond of n-heptane while impeding C–C bond cleavage in the aromatization process.
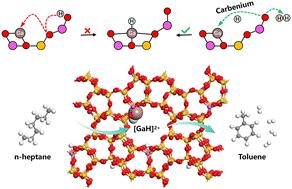
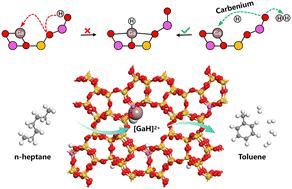
Ga/H-ZSM-5 沸石上活性 [GaH]2+ 位点在正庚烷脱氢环化过程中的关键作用
Ga/H-ZSM-5 沸石一直被认为是轻质烷烃芳香化的有效催化剂。在本研究中,一个三配位硒离子被确定为形成 [GaH]2+ 活性位点的关键中间体。特别是,硒离子作为桥梁的参与促进了[GaH]2+活性位点在动力学上无阻碍地形成和恢复。利用密度泛函理论计算,探索了 H-ZSM-5 和 Ga/H-ZSM-5 沸石的正庚烷综合反应途径,包括通过 C1-C6 闭环转化为甲苯。与 BAS 相比,[GaH]2+ 活性位点大大降低了 C-H 键裂解的活化障碍。此外,Bader 电荷和晶体轨道 Hamilton 群体分析证实,[GaH]2+ 活性位点在芳香化过程中促进了正庚烷 C-H 键的活化,同时阻碍了 C-C 键的裂解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


