Facile synthesis and electronic structure optimization of sub-nanometer palladium clusters for efficient direct synthesis of H2O2†
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Direct synthesis of hydrogen peroxide (H2O2) from hydrogen and oxygen is an atom-efficient and environmentally benign method. However, achieving high H2O2 selectivity and productivity with sub-nanometer Pd clusters remains challenging due to the tendency of undercoordinated surface sites to cleave O–O bonds and form water. Herein, sub-nanometer Pd clusters are confined to ammonia-treated ZIF-8-derived mesoporous carbon (ZDC) with controlled nitrogen configurations, which facilitates the effective direct synthesis of H2O2 by optimizing the electronic structure of Pd atoms via a strong metal–support interaction (SMSI). The sub-nanometer catalyst Pd/ZDC1.0 achieves a remarkable H2O2 selectivity of 81.6% and productivity of 3323 mmol gPd−1 h−1, which are 12.5% and 122.8% higher than those of the sub-nanometer catalyst Pd/XC-72 without nitrogen doping, respectively. Structural characterization shows that the pore structure and pyridine nitrogen content of Pd/ZDCX are proportional to the ammonia treatment time. Theoretical calculations demonstrate that there is a strong electron transfer between sub-nanometer Pd clusters and pyridine nitrogen sites, leading to a downward shift in the d-band center of Pd atoms. The electronic effect weakens the adsorption of reactive intermediates on undercoordinated surface sites and suppresses O–O bond dissociation, contributing to enhanced selectivity and preventing water formation.
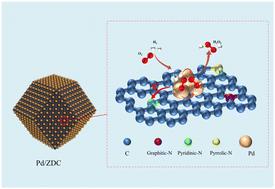
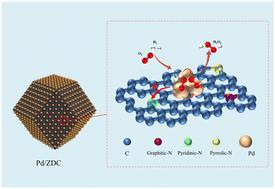
亚纳米钯簇的简易合成和电子结构优化,用于高效直接合成 H2O2
从氢气和氧气直接合成过氧化氢(H2O2)是一种原子高效且对环境无害的方法。然而,由于配位不足的表面位点容易裂解 O-O 键并形成水,因此利用亚纳米级钯团簇实现高 H2O2 选择性和生产率仍具有挑战性。在本文中,亚纳米级 Pd 簇被限制在氨处理过的 ZIF-8 衍生介孔碳(ZDC)中,其氮构型可控,通过强金属-支撑相互作用(SMSI)优化 Pd 原子的电子结构,从而促进 H2O2 的有效直接合成。亚纳米催化剂 Pd/ZDC1.0 的 H2O2 选择性和生产率分别达到 81.6% 和 3323 mmol gPd-1 h-1 ,比未掺氮的亚纳米催化剂 Pd/XC-72 分别高出 12.5% 和 122.8%。结构表征表明,Pd/ZDCX 的孔结构和吡啶氮含量与氨处理时间成正比。理论计算表明,亚纳米钯簇和吡啶氮位点之间存在强烈的电子转移,导致钯原子的 d 带中心下移。电子效应减弱了反应性中间产物在配位不足的表面位点上的吸附,抑制了 O-O 键的解离,从而提高了选择性并防止了水的形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
文献相关原料
公司名称
产品信息
阿拉丁
2-Methylimidazole
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


