Comprehensive understanding of ethylene epoxidation on copper catalysts: a microkinetic study with coverage effects†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
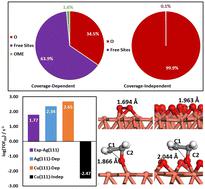
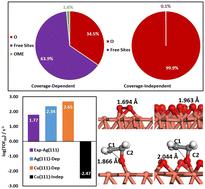
Ethylene epoxidation is one of the fundamental industrial reactions, garnering extensive theoretical and experimental studies. While silver has traditionally been the catalyst of choice for this reaction, copper has received comparatively little attention. In this study, we apply a coverage-dependent microkinetic modeling to quantitatively investigate ethylene epoxidation on Cu(111), serving as a model system to study the intrinsic activity and selectivity of Cu catalysts. The coverage-dependent simulation takes into account both self and cross-interactions of adsorbates, as well as the coverage effects on the transition states of each elementary step. In contrast, the coverage-independent modeling is conducted without considering coverage effects. We observe that the coverage-dependent modelling reveals the Cu(111) surface with coverage exceeding 30% oxygen atoms with a high turnover frequency (log(TOF) = 2.65) at 500 K. In contrast, the coverage-independent results indicate the Cu(111) surface being completely covered by oxygen atoms, leading to detrimental poisoning effects (log(TOF) = −2.47). We show that the EO selectivity on Cu(111) can be at a high level of 80% under all studied conditions in contrast to only ∼40% EO selectivity on Ag(111). Detailed structural analyses unveil the fundamental reasons why Cu catalysts are more selective for ethylene epoxidation. Furthermore, we suggest that reducing temperature and increasing oxygen pressure can effectively improve EO selectivity for industrial ethylene epoxidation.
全面了解铜催化剂上的乙烯环氧化反应:带覆盖效应的微动力学研究
乙烯环氧化反应是基本的工业反应之一,已引起广泛的理论和实验研究。银一直是该反应的首选催化剂,而铜却很少受到关注。在本研究中,我们采用了一种依赖于覆盖率的微动力学模型来定量研究铜(111)上的乙烯环氧化反应,并将其作为研究铜催化剂内在活性和选择性的模型系统。与覆盖率相关的模拟考虑了吸附剂的自相互作用和交叉相互作用,以及覆盖率对每个基本步骤过渡态的影响。相反,与覆盖无关的建模则不考虑覆盖效应。我们观察到,与覆盖率相关的建模显示,在 500 K 时,Cu(111) 表面的氧原子覆盖率超过 30%,且具有较高的周转频率(log(TOF) = 2.65)。我们的研究表明,在所有研究条件下,Cu(111) 表面的环氧乙烷选择性高达 80%,而 Ag(111) 表面的环氧乙烷选择性仅为 40%。详细的结构分析揭示了铜催化剂对乙烯环氧化选择性更高的根本原因。此外,我们还建议降低温度和增加氧压可有效提高工业乙烯环氧化的环氧乙烷选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


