Superacidity in Zr(iv)/Ce(iii) MOF-808: unlocking biodiesel production from microalgae lipids at reduced temperatures†
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
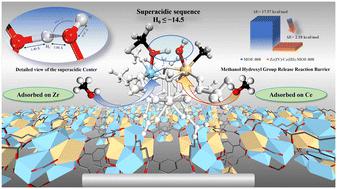
To manage production costs and lower the conversion temperature of microalgal lipids, this study engineered superacid sites in MOF-808, facilitating lipid transformation at markedly reduced temperatures. Through simple substitution and activation steps, secondary building units formed by Zr(iv) and Ce(iii) were assembled with benzene-1,3,5-tricarboxylate ligands, resulting in a unique superacid structure. This superacid structure has a Hammett acidity value of less than −14.5 and a deprotonation energy of 1016.25 kJ mol−1, both lower than those of sulfuric acid. Density functional theory and solid-state NMR analyses confirmed the superacidity arises from a weakly constrained superacid proton within a bridging structure, formed by two methanol molecules attracted to adjacent Ce and Zr atoms. Variable temperature infrared spectroscopy demonstrated the low-temperature activation of methanol, with the optimal reaction temperature reduced from 200 °C to 150 °C and pressure reduced by 2952 kPa. Unlike traditional solid superacids, this methanol-based superacid configuration prevents the loss of superacid sites, maintaining over 97% efficiency after five cycles.
Zr(IV)/Ce(III)MOF-808中的超酸性:利用微藻脂类在低温下生产生物柴油
为了控制生产成本并降低微藻脂质的转化温度,本研究在 MOF-808 中设计了超酸性位点,从而在明显降低温度的条件下促进脂质转化。通过简单的置换和活化步骤,Zr(IV) 和 Ce(III) 形成的二级构建单元与苯-1,3,5-三羧酸配体组装在一起,形成了独特的超酸性结构。这种超酸结构的哈米特酸度值小于-14.5,去质子化能为 1016.25 kJ mol-1,均低于硫酸。密度泛函理论和固态核磁共振分析证实,超酸性来自桥接结构中的一个弱约束超酸质子,该结构由两个甲醇分子吸引到相邻的 Ce 原子和 Zr 原子上形成。变温红外光谱显示了甲醇的低温活化,最佳反应温度从 200 °C 降至 150 °C,压力降低了 2952 kPa。与传统的固体超酸不同,这种基于甲醇的超酸构型可防止超酸位点的损失,在五个循环后仍能保持 97% 以上的效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


