Arsenic trioxide and p97 inhibitor synergize against acute myeloid leukemia by targeting nascent polypeptides and activating the ZAKα–JNK pathway
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
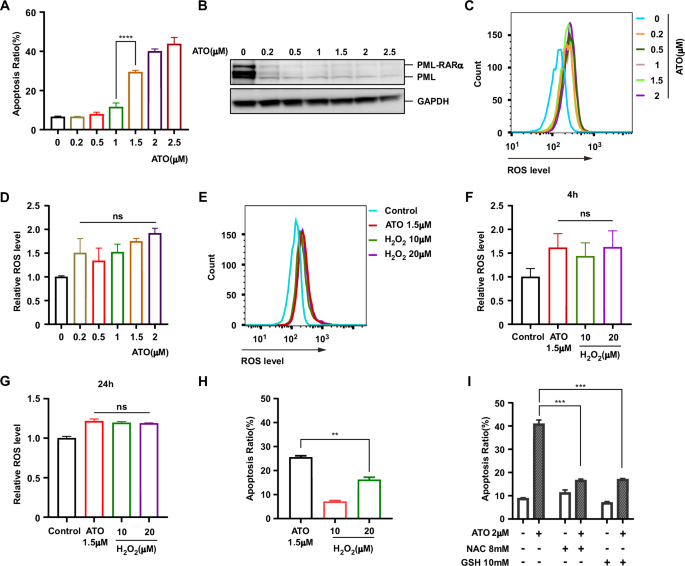
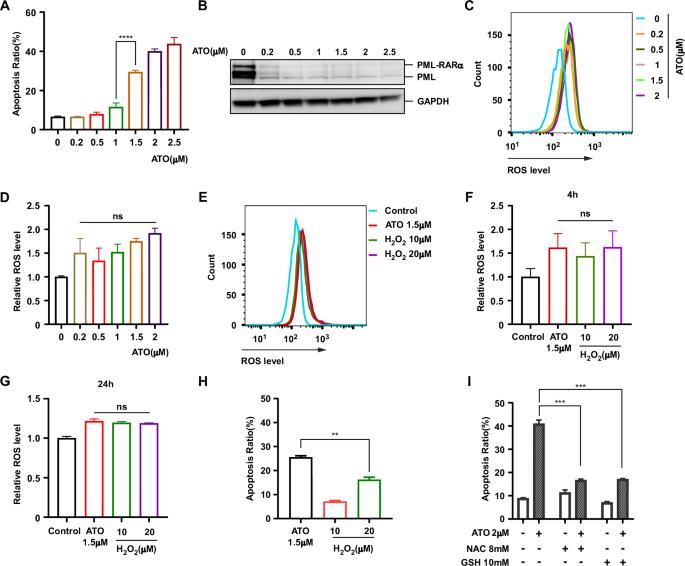
Arsenic trioxide (ATO) has exhibited remarkable efficacy in treating acute promyelocytic leukemia (APL), primarily through promoting the degradation of the PML-RARα fusion protein. However, ATO alone fails to confer any survival benefit to non-APL acute myeloid leukemia (AML) patients and exhibits limited efficacy when used in combination with other agents. Here, we explored the general toxicity mechanisms of ATO in APL and potential drugs that could be combined with ATO to exhibit synergistic lethal effects on other AML. We demonstrated that PML-RARα degradation and ROS upregulation were insufficient to cause APL cell death. Based on the protein synthesis of different AML cells and their sensitivity to ATO, we established a correlation between ATO-induced cell death and protein synthesis. Our findings indicated that ATO induced cell death by damaging nascent polypeptides and causing ribosome stalling, accompanied by the activation of the ZAKα-JNK pathway. Furthermore, ATO-induced stress activated the GCN2–ATF4 pathway, and ribosome-associated quality control cleared damaged proteins with the assistance of p97. Importantly, our data revealed that inhibiting p97 enhanced the effectiveness of ATO in killing AML cells. These explorations paved the way for identifying optimal synthetic lethal drugs to enhance ATO treatment on non-APL AML.
三氧化二砷和 p97 抑制剂通过靶向新生多肽和激活 ZAKα-JNK 通路对急性髓性白血病产生协同作用。
三氧化二砷(ATO)主要通过促进 PML-RARα 融合蛋白的降解来治疗急性早幼粒细胞白血病(APL),疗效显著。然而,单独使用 ATO 无法为非 APL 急性髓性白血病(AML)患者带来任何生存益处,与其他药物联合使用时疗效有限。在此,我们探讨了 ATO 在 APL 中的一般毒性机制,以及与 ATO 联用可对其他 AML 产生协同致死效应的潜在药物。我们证明,PML-RARα降解和ROS上调不足以导致APL细胞死亡。根据不同 AML 细胞的蛋白质合成及其对 ATO 的敏感性,我们建立了 ATO 诱导的细胞死亡与蛋白质合成之间的相关性。我们的研究结果表明,ATO 通过破坏新生多肽并导致核糖体停滞,同时激活 ZAKα-JNK 通路,诱导细胞死亡。此外,ATO诱导的应激激活了GCN2-ATF4通路,核糖体相关质量控制在p97的协助下清除了受损蛋白质。重要的是,我们的数据显示,抑制 p97 能增强 ATO 杀死 AML 细胞的效果。这些探索为确定最佳合成致死药物铺平了道路,以增强ATO对非APL AML的治疗效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


