Small Molecule Topical Ophthalmic Formulation Development—Data Driven Trends & Perspectives from Commercially Available Products in the US
IF 3.7
3区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Topical ophthalmic drug product development is a niche research domain as the drug formulations need to be designed to perform in the unique ocular physiological conditions. The most common array of small molecule drug formulations intended for topical ophthalmic administration include solutions, suspensions, emulsions, gels, and ointments. The formulation components such as excipients and container closure are unique to serve the needs of topical ophthalmic delivery compared to other parenteral products. The selection of appropriate formulation platform, excipients, and container closure for delivery of drugs by topical ophthalmic route is influenced by a combination of factors like physicochemical properties of the drug molecule, intended dose, pharmacological indication as well as the market trends influenced by the patient population. In this review, data from literature and packaging inserts of 118 reference listed topical ophthalmic medications marketed in the US are collected and analyzed to identify trends that would serve as a guidance for topical ophthalmic formulation development for small molecule drugs. Specifically, the topics reviewed include current landscape of the available small molecule topical ophthalmic drug products in the US, physicochemical properties of the active pharmaceutical ingredients (APIs), formulation platforms, excipients, and container closure systems.
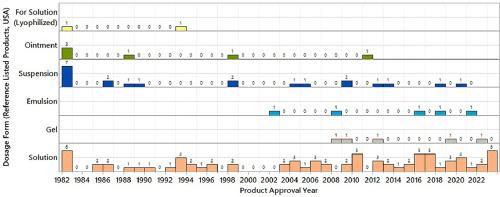
小分子局部眼科制剂的开发--美国商用产品的数据驱动趋势与前景。
眼科局部用药产品开发是一个利基研究领域,因为药物制剂需要在独特的眼部生理条件下发挥作用。最常见的眼科局部用药小分子药物制剂包括溶液剂、悬浮剂、乳剂、凝胶剂和软膏剂。与其他非肠道用药产品相比,眼科局部给药所需的辅料和容器封口等制剂成分是独一无二的。选择合适的制剂平台、辅料和容器封口来通过眼科局部给药途径给药,会受到多种因素的影响,如药物分子的理化性质、预期剂量、药理适应症以及受患者群体影响的市场趋势。在本综述中,我们收集并分析了在美国上市的 118 种眼科局部用药参考文献和包装插页中的数据,以确定可作为小分子药物眼科局部用药制剂开发指南的趋势。具体而言,审查的主题包括美国现有小分子眼科局部用药产品的现状、活性药物成分 (API) 的理化性质、制剂平台、辅料和容器封闭系统。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.30
自引率
13.20%
发文量
367
审稿时长
33 days
期刊介绍:
The Journal of Pharmaceutical Sciences will publish original research papers, original research notes, invited topical reviews (including Minireviews), and editorial commentary and news. The area of focus shall be concepts in basic pharmaceutical science and such topics as chemical processing of pharmaceuticals, including crystallization, lyophilization, chemical stability of drugs, pharmacokinetics, biopharmaceutics, pharmacodynamics, pro-drug developments, metabolic disposition of bioactive agents, dosage form design, protein-peptide chemistry and biotechnology specifically as these relate to pharmaceutical technology, and targeted drug delivery.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


