CCL2 mediated IKZF1 expression promotes M2 polarization of glioma-associated macrophages through CD84-SHP2 pathway
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The proneural-mesenchymal (PN-MES) transformation of glioma stem cells (GSCs) can significantly increase proliferation, invasion, chemotherapy tolerance, and recurrence. M2-like polarization of tumor-associated macrophages (TAMs) has a strong immunosuppressive effect, promoting tumor malignancy and angiogenesis. There is limited understanding on the interactions between GSCs and TAMs as well as their associated molecular mechanisms. In the present study, bioinformatics analysis, GSC and TAM co-culture, determination of TAM polarization phenotypes, and other in vitro experiments confirmed that CCL2 secreted by MES-GSCs promotes TAM-M2 polarization via the IKZF1-CD84-SHP2 pathway and PN-MES transformation of GSCs via the IKZF1-LRG1 pathway in TAMs. IKZF1 inhibitors could significantly reduce tumor volumes in animal glioma models and improve survival, as well as suppress TAM-M2 polarization and the GSC malignant phenotype. The results of this study indicate the important interaction between TAMs and GSCs in the glioma microenvironment as well as its role in tumor progression. The findings also suggest a novel target for follow-up clinical transformation research on the regulation of TAM function and GSCs malignant phenotype.
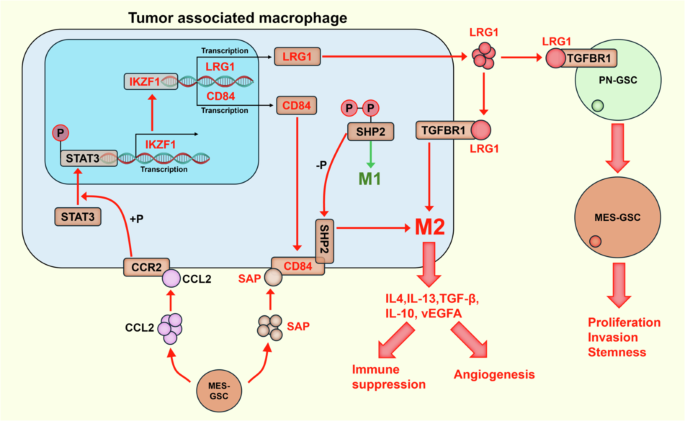
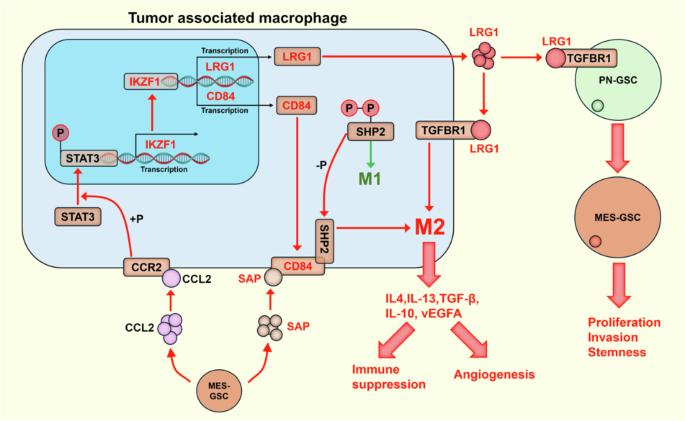
CCL2 介导的 IKZF1 表达通过 CD84-SHP2 通路促进胶质瘤相关巨噬细胞的 M2 极化。
胶质瘤干细胞(GSCs)的腱鞘-间充质(PN-MES)转化可显著增加增殖、侵袭、化疗耐受性和复发。肿瘤相关巨噬细胞(TAMs)的M2样极化具有很强的免疫抑制作用,可促进肿瘤恶变和血管生成。目前对GSCs和TAMs之间的相互作用及其相关分子机制的了解还很有限。本研究通过生物信息学分析、GSC与TAM共培养、TAM极化表型测定等体外实验证实,MES-GSCs分泌的CCL2可通过IKZF1-CD84-SHP2途径促进TAM-M2极化,并通过IKZF1-LRG1途径促进TAMs中GSCs的PN-MES转化。IKZF1抑制剂能显著减少动物胶质瘤模型中的肿瘤体积并提高生存率,还能抑制TAM-M2极化和GSC恶性表型。该研究结果表明了胶质瘤微环境中 TAM 与 GSC 之间的重要相互作用及其在肿瘤进展中的作用。研究结果还为后续临床转化研究TAM功能和GSCs恶性表型的调控提出了新的靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


