The potential effect of romosozumab on perioperative management for instrumentation surgery
Abstract
Background
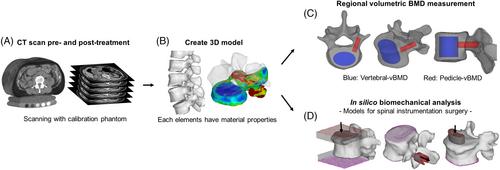
Age-related changes in bone health increase the risk for complications in elderly patients undergoing orthopedic surgery. Osteoporosis is a key therapeutic target that needs to be addressed to ensure successful instrumentation surgery. The effectiveness of pharmacological interventions in orthopedic surgery, particularly the new drug romosozumab, is still unknown. We aim to evaluate the effect of 3-month romosozumab treatment on biomechanical parameters related to spinal instrumentation surgery, using the Quantitative Computed Tomography (QCT)-based Finite Element Method (FEM).
Methods
This open-labeled, prospective study included 81 patients aged 60 to 90 years, who met the osteoporosis criteria and were scheduled for either romosozumab or eldecalcitol treatment. Patients were assessed using blood samples, dual-energy absorptiometry (DXA), and QCT. Biomechanical parameters were evaluated using FEM at baseline and 3 months post-treatment. The primary endpoints were biomechanical parameters at 3 months, while secondary endpoints included changes in regional volumetric bone mineral density around the pedicle (P-vBMD) and vertebral body (V-vBMD).
Results
Romosozumab treatment led to significant gains in P-vBMD, and V-vBMD compared to eldecalcitol at 3 months. Notably, the romosozumab group showed greater improvements in all biomechanical parameters estimated by FEM at 3 months compared to the eldecalcitol group.
Conclusion
Romosozumab significantly increased the regional vBMD as well as biomechanical parameters, potentially offering clinical benefits in reducing post-operative complications in patients with osteoporosis undergoing orthopedic instrumentation surgery. This study highlights the novel advantages of romosozumab treatment and advocates further research on its effectiveness in perioperative management.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


