Fluoxetine accelerates epileptogenesis and magnifies disease severity in a rat model of acquired epilepsy
Abstract
Objective
Many people with epilepsy experience comorbid anxiety and depression, and antidepressants remain a primary treatment for this. Emerging evidence suggests that these agents may modulate epileptogenesis to influence disease severity. Here, we assessed how treatment with the selective serotonin reuptake inhibitor (SSRI) antidepressant fluoxetine impacts epileptogenic, behavioral, and pathological sequelae following status epilepticus.
Methods
Male Wistar rats received kainic acid to induce status epilepticus (SE) or vehicle (sham). Animals then received either fluoxetine (10 mg/kg/day) or vehicle for 8 weeks via subcutaneous osmotic pump. Video-electroencephalography was recorded continuously until behavioral testing at day 56, including assessments of anxiety- and depression-like behavior and spatial cognition. Postmortem immunocytochemistry studies examined mossy fiber sprouting.
Results
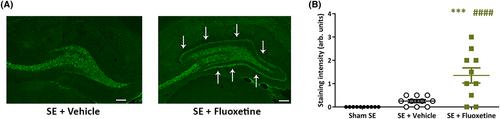
Fluoxetine treatment significantly accelerated epileptogenesis following SE, reducing the average period to the first spontaneous seizure (from 32 days [vehicle] to 6 days [fluoxetine], p < .01). Also, fluoxetine exposure magnified the severity of the resultant epilepsy, increasing seizure frequency compared to vehicle (p < .01). Exposure to fluoxetine was associated with improved anxiety- and depression-like behaviors but significantly worsened cognition. Mossy fiber sprouting was more pronounced in fluoxetine-treated rats compared to vehicle (p < .0001).
Significance
Our studies demonstrate that, using a model exhibiting spontaneous seizures, epileptogenesis is accelerated and magnified by fluoxetine, an effect that may be related to more severe pathological neuroplasticity. The differential influence of fluoxetine on behavior indicates that different circuitry and mechanisms are responsible for these comorbidities. These findings suggest that caution should be exercised when prescribing SSRI antidepressants to people at risk of developing epilepsy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


