BCR::ABL1 Proteolysis-targeting chimeras (PROTACs): The new frontier in the treatment of Ph+ leukemias?
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
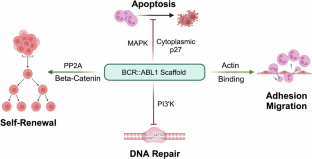
BCR::ABL1 tyrosine kinase inhibitors (TKIs) have turned chronic myeloid leukemia (CML) from a lethal condition into a chronic ailment. With optimal management, the survival of CML patients diagnosed in the chronic phase is approaching that of age-matched controls. However, only one-third of patients can discontinue TKIs and enter a state of functional cure termed treatment-free remission (TFR), while the remainder require life-long TKI therapy to avoid the recurrence of active leukemia. Approximately 10% of patients exhibit primary or acquired TKI resistance and eventually progress to the blast phase. It is thought that recurrence after attempted TFR originates from CML stem cells (LSCs) surviving despite continued suppression of BCR::ABL1 kinase. Although kinase activity is indispensable for induction of overt CML, kinase-independent scaffold functions of BCR::ABL1 are known to contribute to leukemogenesis, raising the intriguing but as yet hypothetical possibility, that degradation of BCR::ABL1 protein may accomplish what TKIs fail to achieve – eliminate residual LSCs to turn functional into real cures. The advent of BCR::ABL1 proteolysis targeting chimeras (PROTACs), heterobifunctional molecules linking a TKI-based warhead to an E3 ligase recruiter, has moved clinical protein degradation into the realm of the possible. Here we examine the molecular rationale as well as pros and cons of degrading BCR::ABL1 protein. We review reported BCR::ABL1 PROTACs, point out limitations of available data and compounds and suggest directions for future research. Ultimately, clinical testing of a potent and specific BCR::ABL1 degrader will be required to determine the efficacy and tolerability of this approach.
BCR::ABL1蛋白水解靶向嵌合体(PROTACs):治疗 Ph+ 白血病的新领域?
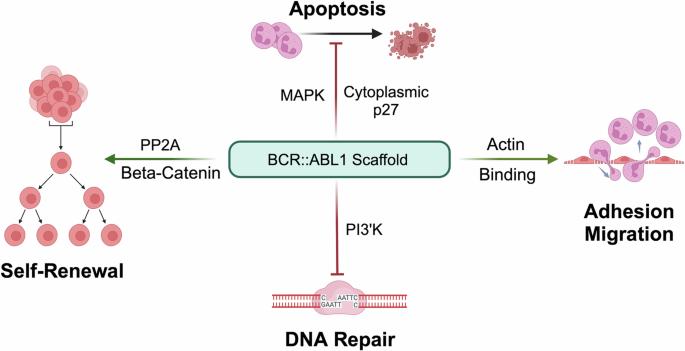
BCR::ABL1酪氨酸激酶抑制剂(TKIs)使慢性髓性白血病(CML)从致命疾病转变为慢性疾病。通过优化治疗,在慢性期确诊的 CML 患者的生存率已接近年龄匹配的对照组。然而,只有三分之一的患者可以停用 TKIs 并进入功能性治愈状态,即无治疗缓解(TFR),其余患者则需要终身接受 TKI 治疗,以避免活动性白血病复发。约有 10% 的患者表现出原发性或获得性 TKI 耐药性,并最终进展到爆发期。有人认为,TFR尝试后的复发源于CML干细胞(LSCs)在BCR::ABL1激酶持续抑制的情况下仍然存活。虽然激酶活性是诱导显性CML所不可或缺的,但已知BCR::ABL1与激酶无关的支架功能有助于白血病的发生,这就提出了一种耐人寻味但尚属假设的可能性,即BCR::ABL1蛋白的降解可能实现TKIs无法实现的目标--消除残留的LSCs,从而将功能性转变为真正的治愈。BCR::ABL1蛋白水解靶向嵌合体(PROTACs)是连接基于TKI的弹头和E3连接酶招募器的异功能分子,它的出现使临床蛋白降解进入了可能的领域。在此,我们研究了降解 BCR::ABL1 蛋白的分子原理和利弊。我们回顾了已报道的 BCR::ABL1 PROTACs,指出了现有数据和化合物的局限性,并提出了未来的研究方向。最终,需要对强效特异性 BCR::ABL1 降解剂进行临床测试,以确定这种方法的疗效和耐受性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


