Novel 2-[(8-hydroxyquinolin-7-yl)(phenyl)methylamino]benzoic acid analogs targeting the active site of botulinum neurotoxins: designing, synthesis, and biological evaluation
Abstract
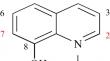
Botulinum neurotoxins are the most lethal and category ‘A’ bioterrorism agent. Despite all efforts, there is no drug available for intoxicating human. The 8-HQ is a well-known privileged scaffold which possesses metal chelation properties and its derived compounds are reported to inhibit the catalytic activity of BoNTs. Novel derivatives of NSC1012 were designed and synthesized via Mannich reaction. After characterization by NMR & mass spectrometry, compounds were studied for its toxicity profiling by in vitro and in vivo experiments. The designed compounds were screened and validated against BoNTs using molecular docking and FTS assay. The derived derivatives displayed no significant hemolytic activity (upto 500 µM) and low cytotoxicity with the CC50 value ranging from 105.94–80.97 µM. The in vivo assay reveals, 25 mM concentration is a NOAEL dose with no observed significant difference in biochemical parameters between the control and treated groups. Molecular docking study showed “hits” with the binding energies for BoNT/A found in the range of −11.65 to −7.24 kcal/mol, BoNT/B between −10.69 to −6.91 kcal/mol, BoNT/E it was −8.18 to −5.30 kcal/mol and for BoNT/F were −8.94 to −6.86 kcal/mol. The FTSA result reveals the binding efficiency of the compounds with the shift in ΔTm from 8.10 to −7.15 °C for serotypes under study. Synthesized compounds are less toxic to the cells, not significantly affect the biochemical profile of the animals, and have shown high binding affinity as well as inhibited the catalytic activity of the BoNTs. These molecules can pave the way for the development of therapeutics against the neurotoxins.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


