Candesartan Cilexetil Formulations in Mesoporous Silica: Preparation, Enhanced Dissolution In Vitro, and Oral Bioavailability In Vivo
IF 3.7
3区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
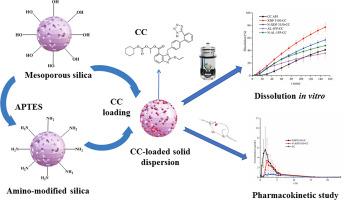
Candesartan cilexetil (CC) is one of well-tolerated antihypertensive drugs, while its poor solubility and low bioavailability limit its use. Herein, two mesoporous silica (Syloid XDP 3150 and Syloid AL-1 FP) and the corresponding amino-modified products (N-XDP 3150 and N-AL-1 FP) have been selected as the carriers of Candesartan cilexetil to prepare solid dispersion through solvent immersion, and characterized through using powder X-ray diffraction analysis, infrared spectroscopy, differential scanning calorimetry, scanning electron microscopy, and solid-state nuclear magnetic resonance spectroscopy, etc. The state of CC changed from crystalline to amorphous after loading onto the silica carriers, in which no interactions between CC and silica existed. Then, the dissolution behaviors in vitro were studied through using flow-through cell dissolution method. CC-XDP 3150 sample exhibited the most extensive dissolution, and the cumulative release of CC from it was 1.88-fold larger than that of CC. Moreover, the pharmacokinetic results in rats revealed that the relative bioavailability of CC-XDP 3150 and CC-N-XDP 3150 solid dispersions were estimated to be 326 % % and 238 % % in comparison with CC, respectively. Clearly, pore size, pore volume, and surface properties of silica carrier have remarkable effect on loading, dissolution and bioavailability of CC. In brief, this work will provide valuable information in construction of mesoporous silica-based delivery system toward poorly water-soluble drugs.
介孔二氧化硅中的坎地沙坦西来替酯制剂:制备、增强体外溶解度和体内口服生物利用度。
坎地沙坦西来替酯(Candesartan cilexetil,CC)是一种耐受性良好的降压药,但其溶解性差、生物利用度低等特点限制了它的使用。本文选择了两种介孔二氧化硅(Syloid XDP 3150和Syloid AL-1 FP)和相应的氨基修饰产品(N-XDP 3150和N-AL-1 FP)作为坎地沙坦西来替昔酯的载体,通过溶剂浸泡法制备固体分散体,并利用粉末X射线衍射分析、红外光谱、差示扫描量热法、扫描电子显微镜和固态核磁共振波谱等对其进行表征。结果表明,CC负载到二氧化硅载体上后,其状态由结晶变为无定形,CC与二氧化硅之间不存在相互作用。然后,通过流动细胞溶解法研究了体外溶解行为。结果表明,CC-XDP 3150样品的溶解度最大,CC的累积释放量是CC的1.88倍。此外,大鼠药代动力学结果显示,CC-XDP 3150 和 CC-N-XDP 3150 固体分散体的相对生物利用度分别为 CC 的 326% 和 238%。显然,二氧化硅载体的孔径、孔容和表面特性对 CC 的负载、溶解和生物利用率有显著影响。简而言之,这项研究将为构建基于介孔二氧化硅的低水溶性药物递送系统提供有价值的信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.30
自引率
13.20%
发文量
367
审稿时长
33 days
期刊介绍:
The Journal of Pharmaceutical Sciences will publish original research papers, original research notes, invited topical reviews (including Minireviews), and editorial commentary and news. The area of focus shall be concepts in basic pharmaceutical science and such topics as chemical processing of pharmaceuticals, including crystallization, lyophilization, chemical stability of drugs, pharmacokinetics, biopharmaceutics, pharmacodynamics, pro-drug developments, metabolic disposition of bioactive agents, dosage form design, protein-peptide chemistry and biotechnology specifically as these relate to pharmaceutical technology, and targeted drug delivery.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


